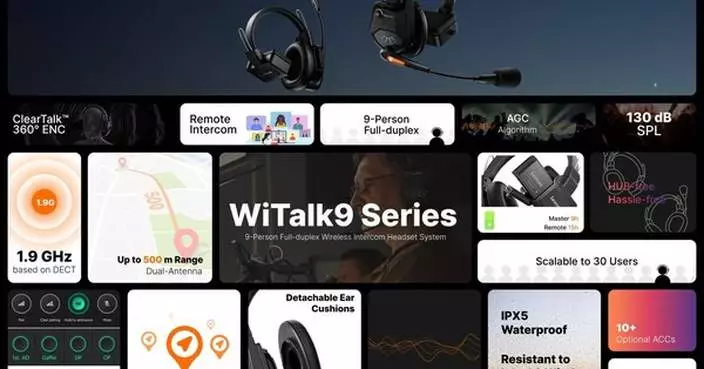
LIVERMORE, Calif., Sept. 3, 2024 /PRNewswire/ -- Grepow, an innovator in battery technology since 1998, today announced a groundbreaking advancement in battery solutions: A new line of metal-cased shaped LiPo batteries. Designed to meet the demanding needs of modern smart wearables and align with upcoming EU regulatory standards, these batteries promise to transform the landscape of mobile power.
Grepow's latest product line features batteries equipped with "stacking" and "pouching" technologies, allowing for unprecedented customization in shape and size. The integration of a durable metal casing enhances the batteries' safety features, allowing them to withstand harsh conditions without compromising performance.
Compliance with Future Regulations:
With an eye towards future requirements, these batteries are engineered to comply with the forthcoming EU Regulations (EU) 2023/1542 on battery removability. This proactive design approach not only ensures ease of battery replacement but also positions Grepow at the forefront of regulatory compliance, offering a competitive edge in the global market.
"Our new metal casing shaped LiPo batteries are tailored to empower next-generation devices with reliability and efficiency," stated the Head of Product Development at Grepow. "With these innovations, we are setting new standards in the battery industry, ensuring our partners and consumers receive the best solutions that technology can offer."
Key Features:
Dedicated to independent research and innovation, Grepow upholds stringent quality standards across its production processes. By utilizing state-of-the-art testing facilities and adhering to ISO13485 standards, the company ensures each product surpasses industry expectations.
About Grepow
Shenzhen Grepow Battery Co., Ltd. is a global leader in the development and manufacturing of rechargeable LiPo batteries, with businesses covering R&D, manufacturing and sales in battery systems. For more information on Grepow's new metal-cased shaped LiPo batteries and to learn more about how they are shaping the future of portable power, please visit https://www.grepow.com.
Contact Information
Email: info@grepow.com
Facebook: https://www.facebook.com/grepowbattery/
YouTube: https://www.youtube.com/c/GrepowBatteryOfficial
LinkedIn: https://www.linkedin.com/company/shenzhen-grepow-battery-co-ltd-/
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Grepow Unveils Cutting-Edge Metal-Cased Shaped LiPo Batteries for Advanced Wearables
SHENZHEN, China, Sept. 15, 2024 /PRNewswire/ -- ImmVira announced the latest clinical results for its lead oncolytic virus product, MVR-T3011, via intravesical administration in patients with high-risk BCG-failure non-muscle invasive bladder cancer (NMIBC). The results were published through poster presentation at the 2024 European Society for Medical Oncology (ESMO 2024).
This Phase I, open-label, dose-escalation and expansion study was conducted in China to assess the safety and efficacy of MVR-T3011 administered intravesically in patients with high-risk BCG-failure NMIBC. The study enrolled a broader patient population, including those with high-grade Ta, T1, or CIS +/- Ta/T1 bladder cancer, regardless of the presence of CIS. This inclusive approach aims to target a wider range of patients, potentially accelerating future trial enrollment and expanding market opportunities. The treatment was delivered via intravesical instillation at three dose levels, with weekly induction course lasting 12 weeks, followed by bi-weekly maintenance course for up to one year.
According to poster presented (cut-off as of June 27, 2024), among 14 evaluable patients, the overall 3-month complete response (CR) rate across all dose levels was 71.4% (10/14). At the 2×109 PFU dose level (expected RP2D), the CR rate reached an impressive 87.5% (7/8). The study showed a favorable safety profile, with no dose-limiting toxicities (DLTs) reported and no maximum tolerated dose (MTD) reached. Additionally, MVR-T3011 treatment simplifies clinical procedure by eliminating the need for bladder prewash by nature, further reducing patient discomfort. The instillation process is both quick and efficient.
By September 15, 2024, the most recent data shows that 20 subjects have received MVR-T3011 treatment. At the RP2D dose level, the 3-month CR rate remained strong at 81.8% (9/11). Of the 9 patients who have reached the 6-month assessment, 8 have maintained CR, while 4 patients have reached the 9-month assessment and all remained in CR. We are continuing to gather additional data to support further analysis.
"There is a significant unmet need for innovative treatments for bladder cancer," said Dr. Grace Zhou, Chairwoman and CEO of ImmVira. "We are highly encouraged by the preliminary safety and efficacy data from this Phase I study, especially the 3-month CR rate over 80%, along with durable responses at 6- and 9-month time points. With this early success, we are committed to accelerating clinical development of intravesical MVR-T3011 treatments for BCG-failure NMIBC patients and exploring its potential across other bladder cancer indications. Our goal is to provide a novel, effective, and well-tolerated treatment solution for patients."
About MVR-T3011
MVR-T3011, ImmVira's proprietary 3-in-1 oHSV, is a novel genetic engineered oHSV with advanced backbone design aiming to achieve the most favorable profile of attenuated HSV-1 with replication potency in tumor cells and highly restricted replication in normal cells, supporting intratumoral, intravenous and intravesical administrations. Additionally, MVR-T3011 incorporated two latest and well-validated exogenous genes, PD-1 antibody and IL-12, to further enhance immune responses in the tumor microenvironment.
About ImmVira
ImmVira is a biotechnology company focused on developing and synthesizing biological vector delivery platform. The company has constructed a fully integrated OVPENS® (Open Vector+ Potent, Enabling, Novel & Safe) platform with solid science, technology and CMC know-how, and three derivative subplatforms including Oncolytic Virus, Cancer Vaccine and Biosynthetic Exosome, to support ongoing R&D, clinical studies and commercialization of best-in-class mono and combo therapies driven by clinical benefits in oncology and non-oncology fields.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
ESMO 2024 - ImmVira Unveils Clinical Results of Intravesical MVR-T3011 for High-Risk BCG-Failure NMIBC Patients
ESMO 2024 - ImmVira Unveils Clinical Results of Intravesical MVR-T3011 for High-Risk BCG-Failure NMIBC Patients

ESMO 2024 - ImmVira Unveils Clinical Results of Intravesical MVR-T3011 for High-Risk BCG-Failure NMIBC Patients