WASHINGTON (AP) — Federal regulators responsible for the safety of the U.S. drug supply are still struggling to get back to where they were in 2019, before the COVID-19 pandemic upended factory inspections in the U.S. and across the world, The Associated Press has found.
An AP analysis of Food and Drug Administration data shows that agency staffers have not returned to roughly 2,000 pharmaceutical manufacturing firms to conduct surveillance inspections since before the pandemic, raising the risks of contamination and other issues in drugs used by millions of Americans.
The firms that are overdue for safety and quality inspections represent about 42% of the 4,700 plants that are currently registered to produce drugs for the U.S. and previously underwent FDA review before May 2019, the AP found. The plants make hundreds of critical medicines, including antibiotics, blood thinners and cancer therapies.
Under FDA’s own guidelines, factories that haven’t been inspected in five or more years are considered a significant risk and are supposed to be prioritized for “mandatory” inspections. Most of the overdue plants are in the U.S., but more than 340 are in India and China, countries that together make up the largest source of drug ingredients used in low-cost U.S. prescriptions.
“Generic drugmakers are under intense pressure to cut their costs and some will do that by cutting quality,” said David Ridley of Duke University, who studies the pharmaceutical industry. “If they’re not inspected, then we won’t know about it until — in a few tragic cases — it’s too late.”
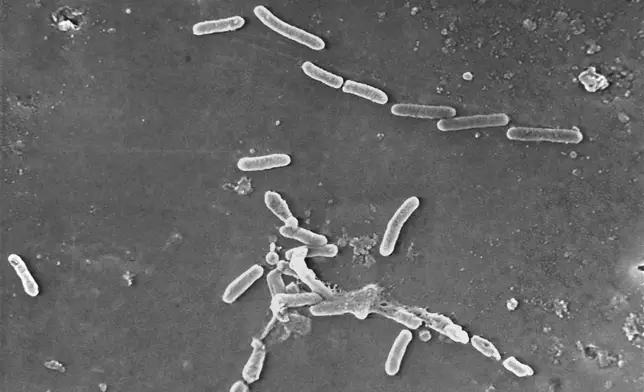
Last year, tainted eyedrops from an Indian factory led to an outbreak of antibiotic-resistant bacteria that sickened more than 80 Americans, killing four of them and blinding more than a dozen others. The plant never registered with the FDA.
Prior to COVID-19, dozens of common medications made at FDA-regulated plants were recalled due to traces of cancer-causing contaminants. The FDA didn’t open its first overseas outposts until 2008, after dozens of U.S. deaths were linked to a contaminated blood thinner imported from a Chinese plant that hadn't been inspected.
“The U.S. drug supply is the safest on the planet and no other regulator conducts more inspections than the FDA,” said FDA Associate Commissioner Michael Rogers, noting that the agency has increased drug inspections each year since 2021 while prioritizing foreign factories.
But last year’s inspection numbers were still down almost 40% from the pre-pandemic period, when the FDA averaged around 4,300 annual inspections. Rogers offered no date for when the backlog of uninspected plants might be cleared.
The agency’s work has been hampered by a wave of staff departures, he said, including longtime inspectors who have found new jobs that often allow them to work from home.
“There’s a significant cost to the agency associated with the loss through attrition of an experienced investigator," Rogers said. "We need to retain these people, and we are.”
The FDA halted all but the most “mission critical” inspections in March 2020. It gradually restarted prioritized inspections later that year, but regular international visits did not resume until 2022.
In a statement, the FDA said that it receives inspection details from international partners, including European regulators, which help the agency decide whether a visit is necessary. The agency also began using video and other online tools to evaluate plants remotely during COVID-19, although those aren't equivalent to physical inspections.
The FDA’s struggles overseeing the global pharmaceutical supply have been documented by the Government Accountability Office, which has flagged the area as a “high risk” issue every year since 2009.
Beginning in the 1990s, drugmakers began shifting manufacturing overseas, first to Puerto Rico, and then to Asia in search of cheaper labor and materials. The FDA has largely been playing catch-up ever since.
“We have to recognize that this is the world we live in and we have to adapt to it,” said Dr. Stephen Ostroff, FDA’s former chief scientist. “That has to include being able to get into these facilities and take a look at what they’re doing, particularly in India and China.”
The FDA keeps a confidential list of drug facilities to inspect, prioritizing them based on potential risks.
The AP created its own list by compiling public records of FDA inspections from before COVID-19 and tracking which firms haven’t received a follow-up within five years. The data accounts for the vast majority of inspections, but has some omissions, including visits required for new drug approvals and those that are part of ongoing government investigations.
The AP's tally of overdue plants also doesn’t include any of the new facilities that have registered with the agency since COVID-19 but haven’t yet had an initial inspection. FDA’s internal list of sites for inspection has increased 14% over the past five years, the agency noted last year.
The FDA said in a statement that it “must be judicious and apply a risk-based approach,” due to the “enormity” of its workload and limited budget.
“We prioritize the inspections that pose the greatest risk to public health,” the agency said.
On a global basis, FDA says only 6% of sites inspected last year had serious problems. But rates are higher in India, where 11% of plants were cited for violations, the most of any country.
Nearly 90% of FDA’s foreign inspections in fiscal 2023 were announced in advance, according to FDA correspondence with Congress obtained via public records requests. The GAO and other government watchdogs have long raised concerns about the practice, which typically gives companies up to 12 weeks to correct or conceal potential problems.
Even with the advanced warnings, the FDA has found egregious violations in overseas factories.
When agency staffers visited Intas Pharmaceuticals’ plant in northwest India for the first time in nearly three years they found what they called a “cascade of failure.” Among the violations, inspectors saw an employee “pouring acetic acid in a trash bin” to destroy company documents used for drug testing. Elsewhere inspectors found “plastic bags filled with torn and discarded” documents relating to quality control measures.
“This kind of behavior has been pointed out to the FDA time and time again by people like me for the last 10 years,” said Dinesh Thakur, a former pharmaceutical industry executive who became a whistleblower for the U.S. government. In 2013, his work led to a $500 million settlement with Indian drugmaker Ranbaxy for falsifying generic drug data.
“If you do not prosecute people who do this kind of wrongdoing, it gives the implicit signal that the FDA will give companies a pass,” Thakur said.
In the case of Intas, FDA issued a warning letter — which has no legal repercussions — and blocked some of the company’s exports to the U.S., while attempting to maintain shipments of the critical chemotherapy drug cisplatin. That strategy backfired in June 2023 when Intas abruptly shuttered the plant, triggering a nationwide shortage of cisplatin, which is used in more than a half-dozen cancers.
FDA inspections in India have been accelerating, but 160 plants are overdue, including some which haven’t been inspected since 2015 yet continue shipping pain pills, antibiotics and other medications to the U.S., according to AP's analysis.
Mexico, France and Spain also have over 100 overdue firms between them.
In China, political tensions have made inspections even more challenging. Just two fulltime FDA inspectors are based in the country, where about 185 factories are overdue. Former FDA officials say the Chinese government has withheld visas from inspectors unless the U.S. reciprocates for Chinese nationals seeking to enter the U.S.
FDA Commissioner Robert Califf has acknowledged his discomfort with the current situation.
“Even if we do periodic inspections, it’s not the same as a society where you have more freedom of information,” Califf told lawmakers at a hearing last year. “I am very concerned about it.”
The FDA could seemingly address its inspection backlog by hiring more investigators or assigning extra work to current staffers. But the agency is struggling to hold onto inspectors who can often earn far more working for the companies they now regulate.
On a call with FDA stakeholders in May, Rogers warned that “our attrition rates and our inability to retain our staff” would soon impact the agency’s ability to oversee drugs and other medical supplies.
The FDA currently has 225 vacancies on its inspection workforce, nearly four times as many as before COVID-19, according to agency records.
New inspectors generally start at an annual salary of about $40,000 and can eventually rise to over $100,000. Job postings alert applicants they may have to travel “up to 50% of the time.”
In 30 years as an FDA inspector, Jose Hernandez experienced firsthand the grueling pace and pressures of the job.
When he began doing international inspections in the mid-1990s, it was on a volunteer basis and employees spent no more than six weeks per year outside the U.S. By the end of his government career, Hernandez was traveling overseas more than four months per year, making multiple trips to Asia.
For each one-week inspection, he said he might need another week or more to write up his report, particularly if he found serious violations. Hernandez says that extra time and attention to detail wasn’t always appreciated by his managers.
“They just wanted people who are like bean counters: walk in, walk out and find nothing so that they can get this report through the system and make their numbers,” he said.
Hernandez retired from the FDA in 2014, earning just over $100,000 in his final year. Today he is a private consultant and says former FDA inspectors can easily earn more than $250,000 working for industry.
“Now I fly business class, I stay in nice hotels, I rest when I get there and nobody tells me what to do,” Hernandez said. “I made the right decision.”
FDA says it's exploring ways to make the jobs more attractive, including extra pay for experienced staffers and offering more flexible, comfortable travel arrangements.
The slowdown in inspections has attracted scrutiny from lawmakers and raised new questions about whether FDA’s decades-old approach is working.
House Republicans have peppered the agency with dozens of inquiries about the program since 2023. In their most recent letter, members of the Energy and Commerce Committee ask regulators to explain why FDA staffers making high-priority inspections in Asia are reporting less than half as many violations today compared with pre-COVID-19.
“The committee is concerned that FDA’s fear of triggering additional drug shortages is driving the decreased rate that FDA issues warning letters,” states the June letter.
FDA’s Rogers rejected the argument, saying inspectors follow strict guidelines when conducting inspections and that decisions about managing drug shortages are handled separately by other parts of the agency.
A pilot program requested by the U.S. Defense Department takes an alternative approach to monitoring the drug supply. The Pentagon is collaborating with a private laboratory, Valisure, to evaluate 40 prescription drugs critical to military personnel, testing them for contaminants, dosing and other issues. Initial findings from the two-year program found serious flaws in about 10% of a subset of drugs tested, according to results shared with the AP.
Laboratory testing of imported drugs has long been standard practice in Europe, where more than 70 private labs operate alongside government regulators.
The testing approach has attracted interest from experts concerned that FDA’s method — rooted in physical inspections and reviewing paperwork — may not be up to the task of uncovering manufacturing problems on the other side of the world.
Stanford University’s Dr. Kevin Schulman says one solution would be to incentivize U.S. pharmacy chains and drug distributors to only purchase drugs from high-quality manufacturers.
Currently, U.S. companies decide which generic drugs to buy based on whichever company has the lowest price, Schulman said. “Purchasers say, ‘Well, it’s the FDA’s job to worry about quality, not ours,’" he said.
Even as the FDA struggles to recover from COVID-19 and reinspect plants worldwide, Schulman sees a potential upside.
“Maybe this is the straw that breaks the camel’s back,” he said. “That, in fact, we should just admit that one U.S. agency can’t regulate the entire global manufacturing for this critically important market.”
Forster reported from New York. Follow Matthew Perrone on X: @AP_FDAwriter
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.

An INTAS pharmaceutical manufacturing plant is seen Ahmedabad, India, Friday, Aug. 2, 2024, where U.S. Food and Drug Administration inspectors documented a “cascade of failure” during a 2022 safety inspection. (AP Photo/Ajit Solanki)

Commuters drive past the INTAS pharmaceutical plant in Ahmedabad, India, Friday, Aug. 2, 2024, where U.S. Food and Drug Administration inspectors documented a “cascade of failure” during a 2022 safety inspection. (AP Photo/Ajit Solanki)

The INTAS pharmaceutical manufacturing plant is seen in Ahmedabad, India, Friday, Aug. 2, 2024, which had produced tainted eyedrops which sickened more than 80 Americans, killing four of them and blinding more than a dozen others. (AP Photo/Ajit Solanki)
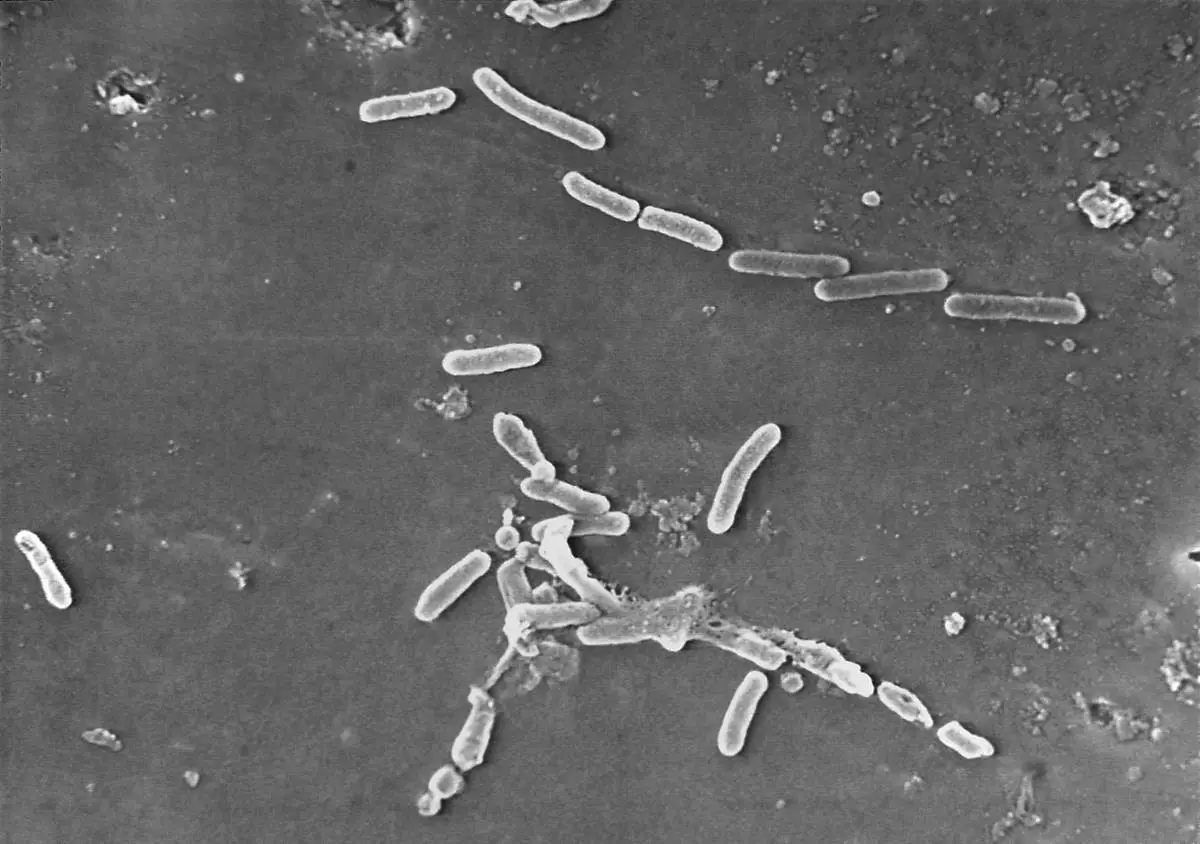
FILE - This scanning electron microscope image made available by the Centers for Disease Control and Prevention shows rod-shaped Pseudomonas aeruginosa bacteria, a drug-resistant strain of which was linked to infections from tainted eyedrops from EzriCare and Delsam Phama which were recalled in February 2023. (Janice Haney Carr/Centers for Disease Control and Prevention via AP, File)

Dr. Stephen Ostroff, the U.S. Food and Drug Administration's former chief scientist, speaks during an interview in Harrisburg, Pa., on Aug. 20, 2024. (AP Photo/Shelby Lum)

FILE - A U.S. Food and Drug Administration building is seen behind FDA logos at a bus stop on the agency's campus in Silver Spring, Md., on Aug. 2, 2018. (AP Photo/Jacquelyn Martin, File)

Commuters drive past the INTAS pharmaceutical manufacturing plant in Ahmedabad, India, Friday, Aug. 2, 2024, which had produced tainted eyedrops which sickened more than 80 Americans, killing four of them and blinding more than a dozen others. (AP Photo/Ajit Solanki)

(AP Illustration/Peter Hamlin)