LONDON (AP) — German submarine-hunting planes will patrol the North Atlantic from a base in Scotland under a new Britain-Germany defense pact sealed in response to the growing threat from Russia.
Defense ministers from Britain and Germany signed the agreement in London on Wednesday in what officials call the first such defense pact between the two NATO member countries to boost European security amid rising Russian aggression.
The deal comes as the war launched by Russia's full-scale invasion of Ukraine in February 2022 grinds on, and with Ukraine and its European allies anxious about the outcome of November's U.S. presidential election.
U.K. Defense Secretary John Healey said the need for Europe to bolster its own security, and for the U.K. to play a bigger role in NATO, “is the driving force behind our NATO-first U.K. defense strategy, (and) behind our reset of U.K. relations with Europe.”
“In a more dangerous world, allies are our strategic strength and we must do more together," Healey said at a news conference in London alongside German Defense Minister Boris Pistorius.
Under the deal, German submarine hunter aircraft are expected to operate “periodically” from a Scottish military base to patrol the North Atlantic. The allies will work closer together to protect vital underwater cables in the North Sea.
The two countries say they will also cooperate to prioritize developing long-range strike weapons that can travel farther than the U.K.'s existing Storm Shadow missiles. German defense giant Rheinmetall is also expected to open a factory producing artillery gun barrels using British steel.
Officials say the pact will mean British and German forces committed to NATO in Estonia and Lithuania will exercise and operate together more closely, ensuring that “land forces on NATO’s eastern flank remain a strong deterrent and are ready to fight and win if required.”
Britain and Germany are also expected to collaborate on developing new land-based and aerial drones.
“We must not take security in Europe for granted,'' Pistorius said. “Russia is waging war against Ukraine, it is increasing its weapons production immensely and has repeatedly launched hybrid attacks on our partners in eastern Europe.
“With the Trinity House Agreement, we are showing that the NATO allies have recognized what these times require and are determined to improve their deterrence and defense capabilities.”

Defence Secretary John Healey speaks to Brigadier Jonathan Sear before his German counterpart Boris Pistorius arrives to sign a new UK-Germany Defence Agreement at Trinity House in London, Wednesday Oct. 23, 2024. (Jordan Pettitt/PA via AP)

Defence Secretary John Healey speaks to members of the Coldstream Guards before his German counterpart Boris Pistorius arrives to sign a new UK-Germany Defence Agreement at Trinity House in London, Wednesday Oct. 23, 2024. (Jordan Pettitt/PA via AP)
LONDON (AP) — Britain's drug regulator approved the Alzheimer's drug Kisunla on Wednesday, but the government won't be paying for it after an independent watchdog agency said the treatment isn't worth the cost to taxpayers.
It is the second Alzheimer's drug to receive such a mixed reception within months. In August, the U.K. regulator authorized Leqembi while the same watchdog agency issued draft guidance recommending against its purchase for the National Health Service.
In a statement on Wednesday, Britain's Medicines and Healthcare regulatory Agency said Kisunla “showed some evidence of efficacy in slowing (Alzheimer's) progression” and approved its use to treat people in the early stages of the brain-robbing disease. Kisunla, also known as donanemab, works by removing a sticky protein from the brain believed to cause Alzheimer’s disease.
Meanwhile, the National Institute for Health and Care Excellence, or NICE, said more evidence was needed to prove Kisunla's worth — the drug's maker, Eli Lilly, says a year's worth of treatment is $32,000. The U.S. Food and Drug Administration authorized Kisunla in July. The roll-out of its competitor drug Leqembi has been slowed in the U.S. by spotty insurance coverage, logistical hurdles and financial worries.
NICE said that the cost of administering Kisunla, which requires regular intravenous infusions and rigorous monitoring for potentially severe side effects including brain swelling or bleeding, “means it cannot currently be considered good value for the taxpayer.”
Experts at NICE said they “recognized the importance of new treatment options” for Alzheimer's and asked Eli Lilly and the National Health Service “to provide additional information to address areas of uncertainty in the evidence.”
Under Britain's health care system, most people receive free health care paid for by the government, but they could get Kisunla if they were to pay for it privately.
“People living with dementia and their loved ones will undoubtedly be disappointed by the decision not to fund this new treatment,” said Tara Spires-Jones, director of the Centre for Discovery Brain Sciences at the University of Edinburgh. “The good news that new treatments can slow disease even a small amount is helpful," she said in a statement, adding that new research would ultimately bring safer and more effective treatments.
Fiona Carragher, chief policy and research officer at the Alzheimer's Society, said the decision by NICE was “disheartening,” but noted there were about 20 Alzheimer's drugs being tested in advanced studies, predicting that more drugs would be submitted for approval within years.
“In other diseases like cancer, treatments have become more effective, safer and cheaper over time,” she said. “ We hope to see similar progress in dementia."
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
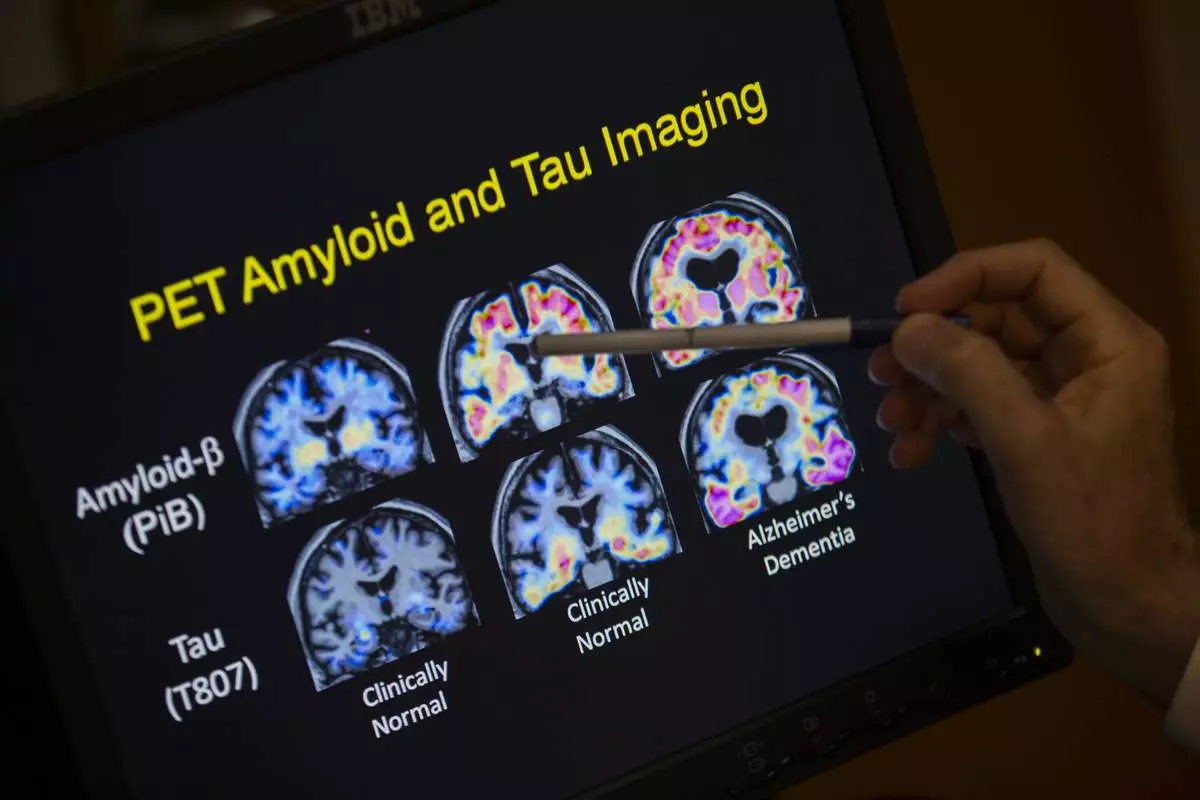
FILE - R. Scott Turner, Professor of Neurology and Director of the Memory Disorder Center at Georgetown University Hospital, points to PET scan results that are part of a study on Alzheimer's disease at Georgetown University Hospital, in Washington, May 19, 2015. (AP Photo/Evan Vucci, File)












