MILPITAS, Calif., Nov. 12, 2024 /PRNewswire/ -- Worldwide silicon wafer shipments increased 5.9% quarter-over-quarter to 3,214 million square inches (MSI) in the third quarter of 2024 and registered 6.8% growth from the 3,010 million square inches recorded during the same quarter last year, the SEMI Silicon Manufacturers Group (SMG) reported in its quarterly analysis of the silicon wafer industry.
"The third quarter wafer shipment results continued the upward trend which started in the second quarter of this year," said Lee Chungwei (æŽå´‡å‰), Chairman of SEMI SMG and Vice President and Chief Auditor at GlobalWafers. "Inventory levels have declined throughout the supply chain but generally remain high. Demand for advanced wafers used for AI continues to be strong. However, the silicon wafer demand for automotive and industrial uses continues to be muted, while the demand for silicon used for handset and other consumer products has seen some areas of improvement. As a result, 2025 is likely to continue upward trends, but total shipments are not yet expected to return to the peak levels of 2022."
Data cited in this release include polished silicon wafers, including those used as virgin test wafers, as well as epitaxial silicon wafers, and non-polished silicon wafers shipped by the wafer manufacturers to end users.
Silicon wafers are the fundamental building material for the majority of semiconductors, which are vital components of all electronic devices. The highly engineered thin disks are produced in diameters of up to 300 mm and serve as the substrate material on which most semiconductors are fabricated.
The SMG is a sub-committee of the SEMI Electronic Materials Group (EMG) and is open to SEMI members involved in manufacturing polycrystalline silicon, monocrystalline silicon or silicon wafers (e.g., as cut, polished, epi). The SMG facilitates collective efforts on issues related to the silicon industry including the development of market information and statistics about the silicon industry and the semiconductor market.
For more information, visit SEMI Worldwide Silicon Wafer Shipment Statistics.
About SEMI
SEMI® is the global industry association connecting over 3,000 member companies and 1.5 million professionals worldwide across the semiconductor and electronics design and manufacturing supply chain. We accelerate member collaboration on solutions to top industry challenges through Advocacy, Workforce Development, Sustainability, Supply Chain Management and other programs. Our SEMICON® expositions and events, technology communities, standards and market intelligence help advance our members' business growth and innovations in design, devices, equipment, materials, services and software, enabling smarter, faster, more secure electronics. Visit www.semi.org, contact a regional office, and connect with SEMI on LinkedIn and X to learn more.
Association Contact
Samer Bahou/SEMI
Phone: 1.408.943.7870
Email: sbahou@semi.org
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Worldwide Silicon Wafer Shipments Increase 6% in Q3 2024, SEMI Reports
PISCATAWAY, N.J., Nov. 13, 2024 /PRNewswire/ -- The global cell and gene therapy (CGT) industry is poised for remarkable growth, driven by significant advances in life sciences and robust investments from capital markets. To strengthen this vital sector, GenScript is bringing together leading scientists, industry experts, investors, and regulatory authorities at the GenScript Biotech Global Forum. This series of conferences aims to address key industrial trends in cell therapy and gene therapy.
For more information, please visit the forum website.
Scan QR code or click the link to register: https://bit.ly/477v0pa
(Register using code GSOM to reserve your free pass.)
In this November 20, GenScript Biotech Global Forum will take place in London during the Jefferies London Healthcare Conference. This year's forum will explore the theme "Unlock the Full Potential of Cell and Gene Therapies." This event aims to foster collaboration among industry leaders while tackling the challenges and opportunities in the CGT landscape.
Highlights of the Agenda Include
Navigating Challenges: Insights into the Future of CGT and Multiple Cell Therapy Technologies in Development
In recent years, cell and gene therapy has become a key player in biopharmaceuticals, with a growing number of approvals annually. CAR-T therapies are the most advanced, while innovations have led to a wider range of cell therapies, including CAR-NK, CAR-M, and TIL, addressing various challenging diseases.
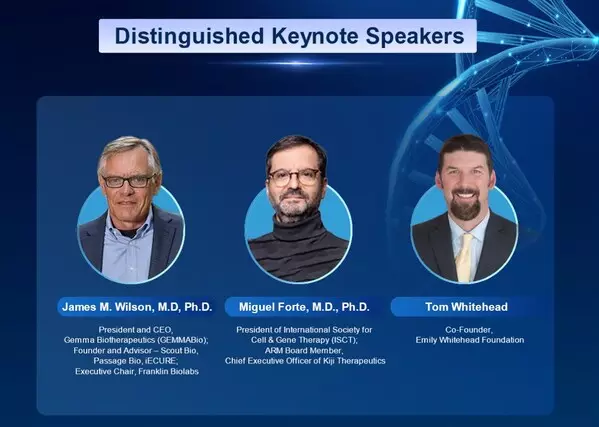
However, the diverse landscape of new therapies and technologies presents numerous challenges. The forum will feature keynote speeches from prominent figures in cell and gene therapy, including Dr. James M. Wilson, President and CEO of Gemma Biotherapeutics; Dr. Miguel Forte, President of the International Society for Cell & Gene Therapy (ISCT) and CEO of Kiji Therapeutics, and Tom Whitehead, co-founder of the Emily Whitehead Foundation will also give a speech to share the life-saving stories of CAR-T therapy.
In-Depth Discussion on Gene Therapy, mRNA Vaccines and the CMC Challenges in Developing and Manufacturing CGT Product
The development of gene therapy and mRNA vaccine technologies has been transformative, particularly during the COVID-19 pandemic, and holds immense promise for treating rare diseases and genetic disorders. As advancements in gene editing reshape clinical applications, emerging innovations in gene therapy and mRNA vaccines are poised to revolutionize medicine. However, the transition of these technologies from research and development to clinical application brings significant challenges. Throughout the development and production of cell and gene therapies, issues related to CMC are prevalent. The quality of raw materials and the stability of supply chains directly impact early development, while the complexity and consistency of manufacturing processes, from small-scale trials to large-scale commercialization, remain key obstacles. Further optimization is needed in process scaling and standardization, alongside the ongoing challenge of ensuring product quality and safety in compliance with stringent regulatory standards for successful market entry. The forum will explore these challenges, as well as emerging market trends and future opportunities for innovation, through in-depth panel discussions.
Exploring the Investment Potential of CGT: A Future Outlook
The cell and gene therapy (CGT) industry faces challenges in investment returns due to high development costs, regulatory hurdles, and manufacturing complexities. However, significant opportunities exist in gene editing and cell-based therapies, which offer promise for treating various diseases. Attracting funding requires understanding the potential impact of these therapies and their path to commercialization. This forum will feature a fireside chat with investment insights, helping attendees explore CGT's potential and how to secure long-term returns as therapies progress toward commercialization.
For more information, please visit the forum website.
Scan QR code or click the link to register: https://bit.ly/477v0pa
(Register using code GSOM to reserve your free pass.)
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Join the GenScript Biotech Global Forum in London to Navigate the Future of Cell and Gene Therapies.
Join the GenScript Biotech Global Forum in London to Navigate the Future of Cell and Gene Therapies.

Join the GenScript Biotech Global Forum in London to Navigate the Future of Cell and Gene Therapies.

Join the GenScript Biotech Global Forum in London to Navigate the Future of Cell and Gene Therapies.

Join the GenScript Biotech Global Forum in London to Navigate the Future of Cell and Gene Therapies.













