DH investigates illegal online sale of slimming products containing controlled drug ingredients
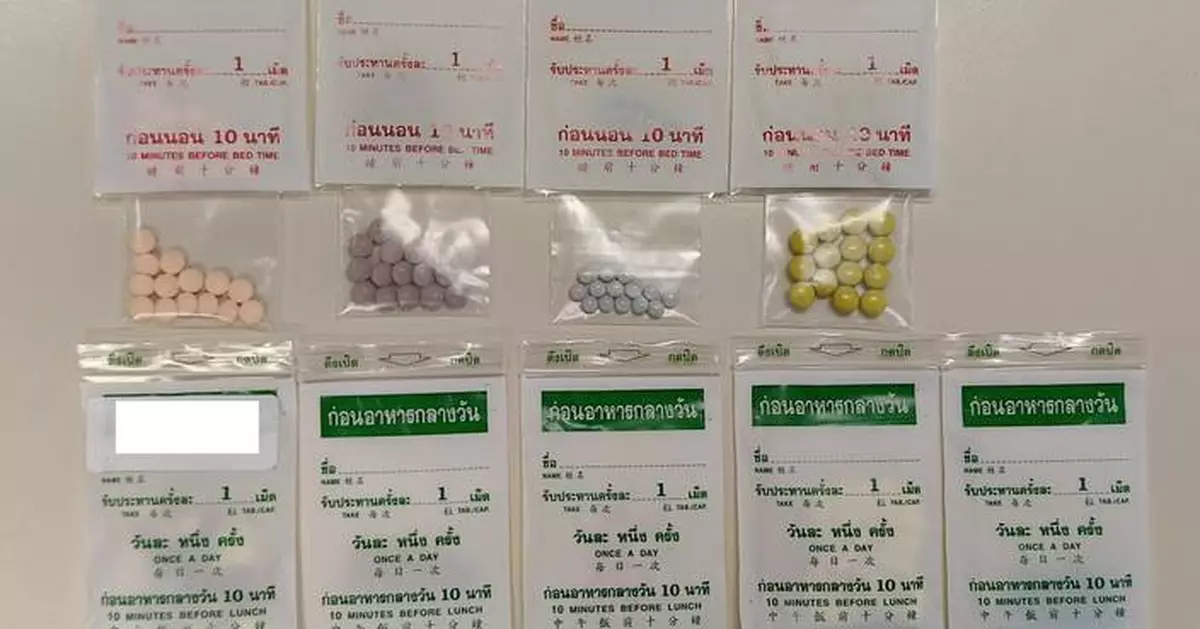
The Department of Health (DH) today (March 27) is investigating a case of illegal sale of slimming products containing undeclared controlled drug ingredients on the Internet and urged the public not to buy or consume the products concerned (see photo).
Acting upon intelligence, the DH purchased on a social media platform some slimming products, reportedly obtained from overseas, for analysis. The laboratory test results revealed that the samples of some products contained frusemide, metformin and thyroxine. All of them are Part 1 poisons under the Pharmacy and Poisons Ordinance (Cap. 138) (PPO).
The packages of the products concerned have Thai, Chinese and English characters, and are suspected to be unregistered pharmaceutical products. The DH will continue to follow up and investigate the case.
Frusemide is used for the treatment of heart diseases and its side effects include low blood pressure andelectrolyteimbalance. Metformin is used for the treatment of diabetes mellitus and its side effects include nausea and diarrhoea. Thyroxine is used for the treatment of hypothyroidism and its side effects include arrhythmia and hypertension. Medicines containing these ingredients should be used under a doctor's directions and be supplied on the premises of an Authorized Seller of Poisons (i.e. pharmacy) under the supervision of a registered pharmacist upon a doctor's prescription.
According to the PPO, all pharmaceutical products must be registered with the Pharmacy and Poisons Board of Hong Kong before they can be sold in the market. Illegal sale or possession of unregistered pharmaceutical products or Part 1 poisons are criminal offences. The maximum penalty for each offence upon conviction is a fine of $100,000 and two years' imprisonment.
The DH strongly urged members of the public not to buy or consume products of doubtful composition or from unknown sources. All registered pharmaceutical products should carry a Hong Kong registration number on the package in the format of "HK-XXXXX". The safety, quality and efficacy of unregistered pharmaceutical products are not guaranteed.
People who have purchased the products concerned should stop consuming them immediately and consult healthcare professionals if in doubt or if they feel unwell after consumption. They may submit the products to the Drug Office of the DH at Room 1804-06, 18/F, Wing On Kowloon Centre, 345 Nathan Road, Kowloon, during office hours for disposal.
Weight control should be achieved through a balanced diet and appropriate exercise. The public should consult healthcare professionals before consuming any medication for weight control. They may visit the website of the Drug Office of the DH for "Health message on overweight problem and slimming products" and "Slimming products with undeclared Western drug ingredients" for information.

DH investigates illegal online sale of slimming products containing controlled drug ingredients Source: HKSAR Government Press Releases
Speech by SITI at Laservall - "From Hong Kong To Global: The Future of Advanced Manufacturing and Microelectronics"
Following is the speech by the Secretary for Innovation, Technology and Industry, Professor Sun Dong, at the Laservall -"From Hong Kong To Global: The Future of Advanced Manufacturing and Microelectronics" event today (March 31):
Ms Lee (Korean Consul (Commerce), Consulate General of the Republic of Korea in Hong Kong, Ms Jaeyon Lee), James (Chairman of Laservall, Mr James Kang), Andrew (Chief Executive Officer of Laservall, Mr Andrew Kim), Albert (Chief Executive Officer of the Hong Kong Science and Technology Parks Corporation, Mr Albert Wong), distinguished guests, ladies and gentlemen,
Good morning. It is my pleasure to stand before you today to celebrate Laservall's global expansion in Hong Kong - a milestone that marks the company's distinctive success achieved in developing advanced high-precision laser inter-connect solutions and automation system for advanced manufacturing.
Founded in Hong Kong in 2003 and commencing its amazing journey at the Hong Kong Science Park in 2024, a cradle that nurtures thousands of innovation and technology (I&T) companies, Laservall has evolved into a world leader in the advanced manufacturing industry today. With its extensive clientele including some renowned global brands in the industry of smartphone camera modules, electronics and automotive, Laservall continues to expand its operations with R&D (research and development) centres and production facilities across Asia.
We are so excited to witness Laservall's success today, as advanced manufacturing has been a pivotal sector and significant power engine to drive the development of new industrialisation in Hong Kong.
Following the promulgation of the Hong Kong Innovation and Technology Development Blueprint in December 2022, the Hong Kong SAR Government has been actively promoting new industrialisation through supporting the advanced manufacturing industries and encouraging the upgrading and transformation of traditional manufacturing sector through the use of I&T, with the aim of driving sustainable economic growth and creating high-quality employment opportunities.
To bolster the development of new industrialisation, our Government has stepped up its efforts by implementing a series of supporting policies and measures. For example, the New Industrialisation Funding Scheme was enhanced to reinforce our efforts in supporting enterprises to adopt smart manufacturing. As of February 2025, the scheme had supported more than 100 new smart production lines. To extend our dedication, we launched the New Industrialisation Acceleration Scheme last year to provide funding support for enterprises engaging in industries of strategic importance. We will also earmark another $100 million for the Pilot Manufacturing and Production Line Upgrade Support Scheme to provide financial support to companies setting up smart production facilities in Hong Kong.
Today, Laservall announces its plan of investing $300 million over the next five years and doubling the size of R&D team by 2026, and extending their R&D team eventually by sixfold by 2028. This not only reflects the company's commitment to innovation but also its unwavering confidence in Hong Kong's I&T industry. Its expansion at the Science Park is a perfect example of how it is leveraging Hong Kong's unique role as a "super-connector" and a "super value-adder" to strengthen its presence in the global market.
May I once again extend my sincere congratulations to Laservall for its outstanding I&T achievement. I am confident that we will soon witness more local companies, like Laservall, stepping onto the global I&T stage and demonstrating the remarkable outcomes of Hong Kong's R&D capabilities to the world in the near future. Thank you.