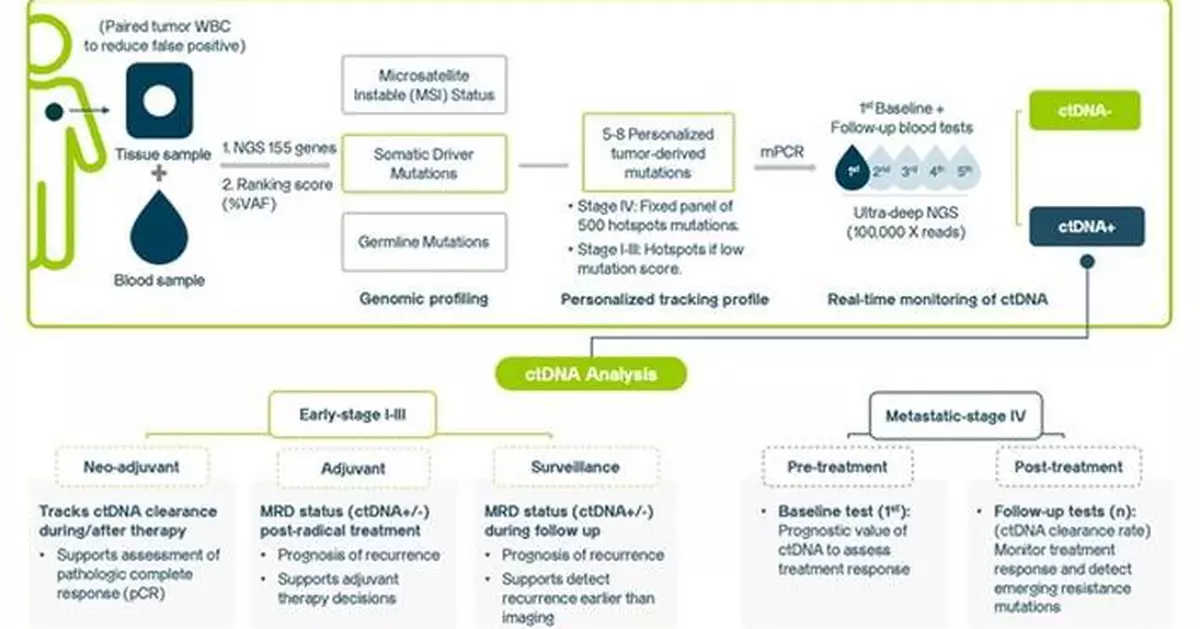
A landmark study recently published in March 2025 by JCO Oncology Advances, demonstrates the potential of K-TRACKTM in monitoring treatment response and assessing recurrence risk among 623 Solid-Tumor Patients of six cancer types (lung, colorectal, breast, gastric, liver, or ovarian cancer).(1)
SINGAPORE, April 19, 2025 /PRNewswire/ -- Gene Solutions, a biotechnology company dedicated to advancing precision oncology in Asia, is excited to announce the publication of a pioneering real-world study in JCO Oncology Advances, validating circulating tumor DNA (ctDNA) as a strong prognostic biomarker to effectively monitor patients during cancer management.
K-TRACKTM is a breakthrough in affordable tumor profiling and personalized cancer monitoring leveraging ctDNA technology. The study, the first of its kind in Asia, confirms K-TRACKTM's clinical value across both early and advanced stages of cancer. The test can detect residual cancer cells (Minimal Residual Disease – MRD) after radical treatment, predict recurrence months earlier than traditional methods, and monitor treatment responses effectively. This innovative dual assay offers hope to patients in underserved regions where access to advanced diagnostic technologies remains a challenge.
Asia represents nearly 50% of global cancer cases, with incidence rates rising at an alarming pace. While some developed markets in the region have seen improvements in cancer mortality rates, Southeast Asia continues to experience high death rates, predominantly driven by cancer recurrence. (2,3)
Research has demonstrated the clinical prognostic value of ctDNA in a variety of cancer types both before and after treatment (4). Despite this potential, the adoption of ctDNA testing remains limited due to factors such as high costs, the absence of standardized methods and insufficient clinical utility of real-world data.
K-TRACKTM was developed as an affordable, reliable personalized test for monitoring ctDNA across multiple solid tumors. It is designed to facilitate early detection of cancer recurrence and provide real-time monitoring of treatment responses. Validated in both clinical trials and real-world studies, K-TRACKTM is built for meaningful impact in cancer care while ensuring accessibility. Here's how we make it work:
Real-world monitoring of ctDNA has proven effective in predicting early cancer recurrence, allowing for timely interventions and improved patient outcomes. In both early and advanced-stage cancer patients, K-TRACKTM has demonstrated high accuracy in predicting recurrence. Following surgery, 84.4% [CA1] of patients with ctDNA-positive results eventually relapsed, while an impressive 96.3% [CA2] of those with ctDNA-negative results remained cancer-free. Moreover, the test could detect relapse up to 5.5-19.5 months earlier than clinical diagnosis by imaging methods. Simultaneously, the six case studies presented illustrated the clinical utility of ctDNA monitoring in predicting treatment response, informing adjuvant chemotherapy decisions, and identifying new resistant mutations to current targeted treatment. Thus, empowering clinicians with real-time, actionable insights to inform treatment decisions.
A Vision for Equitable Care
"Access to cutting-edge, personalized cancer treatment and monitoring—based on tumor genomic profiling— too often depends on where you live or what you can afford," said Dr. Lan N. Tu, Principal Investigator at Gene Solutions. "Our mission with K-TRACKTM is to close that gap by providing an affordable and precise real-time solution for routine clinical use."
Gene Solutions is actively investing in two key areas:
1. On-going Clinical Trials and Real-World Studies:
The company is conducting comprehensive research to validate the role of ctDNA across various cancer types and treatment stages. This includes evaluating responses to TKIs and immune checkpoint inhibitors (ICIs) in lung cancer, neoadjuvant therapy in breast cancer, total neoadjuvant therapy in rectal cancer, and the use of biomarkers to inform immunotherapy treatment decisions in advanced cancers.
2. Biopharma Collaborations:
Gene Solutions is actively pursuing partnerships with biopharmaceutical companies to provide innovative solutions aimed at accelerating drug development across a range of therapeutic modalities. As the scientific evidence supporting ctDNA continues to grow, Gene Solutions is committed to expanding its research and fostering collaborations with current and new partners to explore new applications in cancer care. The company's direction aligns with the FDA's recommendation on ctDNA use as a biomarker in the development of curative-intent therapies for solid tumors which states: "ctDNA as a biomarker has a number of potential regulatory and clinical uses in the early-stage setting that may assist and expedite drug development." (5)
About Gene Solutions
Gene Solutions, a multinational biotech company in Asia, is leading the way in leveraging advanced AI and ctDNA technologies for innovative cancer detection solutions. The company partners with over 4,500 hospitals and clinics across Southeast Asia and employs a dedicated team of approximately 250 biology experts and technicians out of a total of 700 employees.
With more than 50 peer-reviewed publications and over 50 multi-center studies conducted across the region, Gene Solutions is recognized for its proprietary research and CAP-accredited next-generation sequencing (NGS) laboratories in Singapore and Vietnam. By integrating multi-dimensional genomics with AI-driven methodologies, the company is committed to transforming cancer care and improving patient outcomes.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Asia's First Real-World ctDNA-MRD Study Strengthens Evidence for Cost-Effective Cancer Monitoring

Asia's First Real-World ctDNA-MRD Study Strengthens Evidence for Cost-Effective Cancer Monitoring