KYOTO, Japan--(BUSINESS WIRE)--Apr 22, 2025--
Kyocera Corporation (President: Hideo Tanimoto; “Kyocera”) announced today that HAPTIVITY ®, its proprietary tactile technology that replicates realistic touch sensations, has been integrated into the Sigma BF, a full-size mirrorless camera to be released this month. This is the first application of HAPTIVITY ® in a mirrorless camera.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250421608405/en/
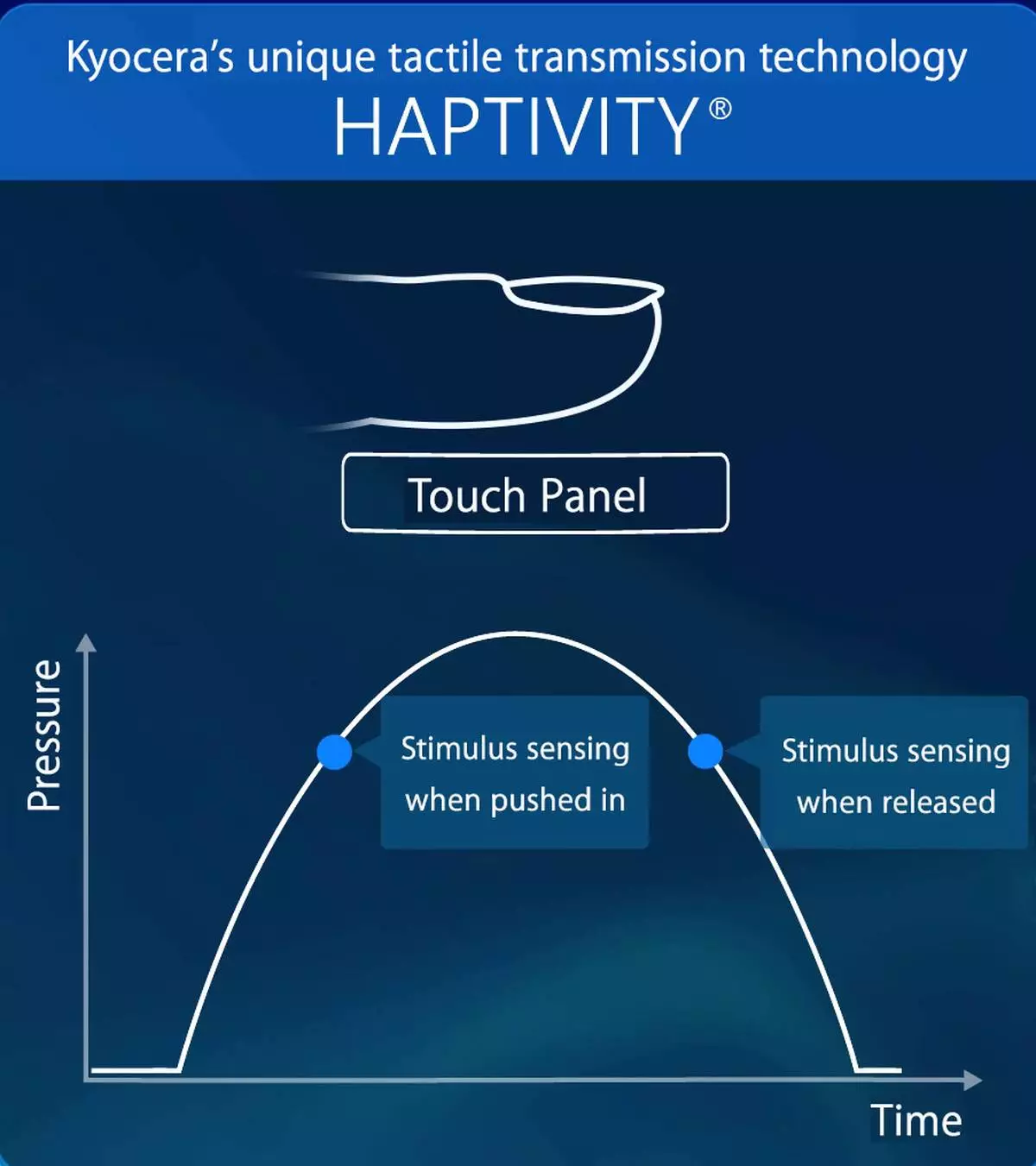
HAPTIVITY® is a groundbreaking technology that harnesses Kyocera’s piezoelectric ceramic vibrating elements to create truly immersive tactile sensations. Biomechanically engineered vibration waveforms provide tactile feedback by stimulating neural receptors in the user’s fingertip, providing physical verification when a button is depressed and released — or even simulating the feel of a physical button where none exists.
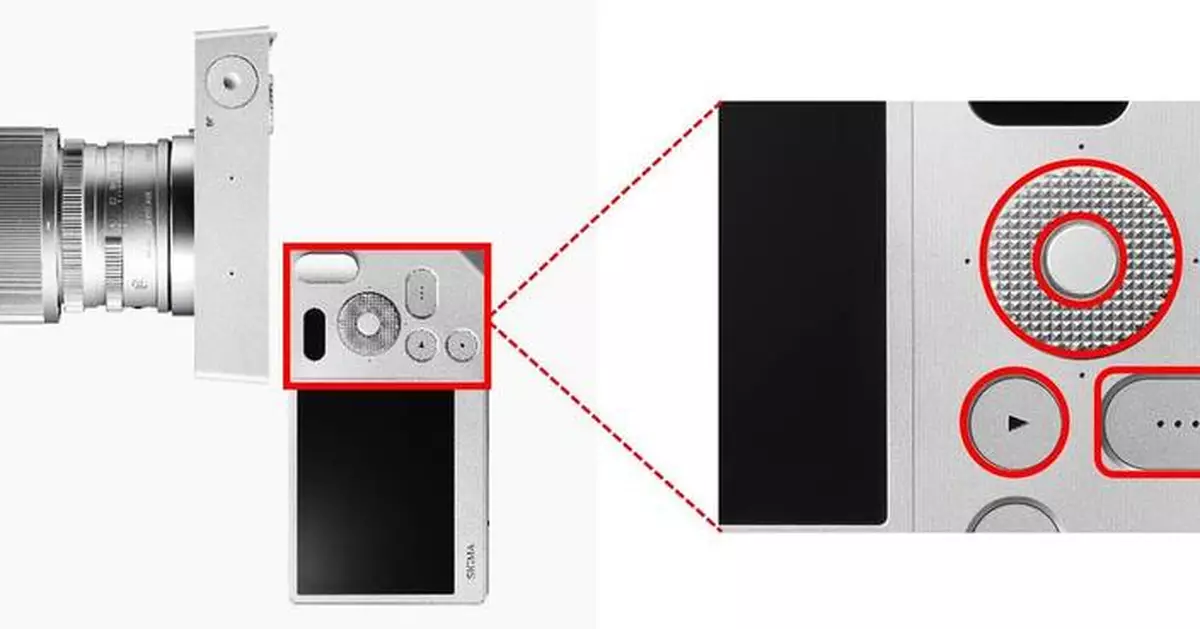
Kyocera’s HAPTIVITY® technology integrates seamlessly into Sigma BF’s three recessed rear buttons (Function Dial, Options, and Playback — including the dial’s up, down, left, and right touchpoints), for accurate, comfortable, intuitive operation. Haptic feedback ensures reliable performance by enhancing user confidence and avoiding duplicate commands.
Kyocera envisions a future where HAPTIVITY® becomes a ubiquitous, high-performance tactile technology. With its piezoelectric ceramic vibrating elements, HAPTIVITY® has the potential to revolutionize consumer and automotive applications as an essential component of the next-generation human-machine interface. It can improve the user experience for any device having physical buttons, or having screens and panels designed to provide tactile feedback.
*HAPTIVITY is a registered trademark of Kyocera Corporation. Kyocera's patented tactile transmission technology optimally controls pressure detection, reproduction of the feeling of pressing a mechanical button, and vibration activation using a combination of piezoelectric elements, vibration amplification mechanisms, control circuits, and software.
Features of HAPTIVITY ®
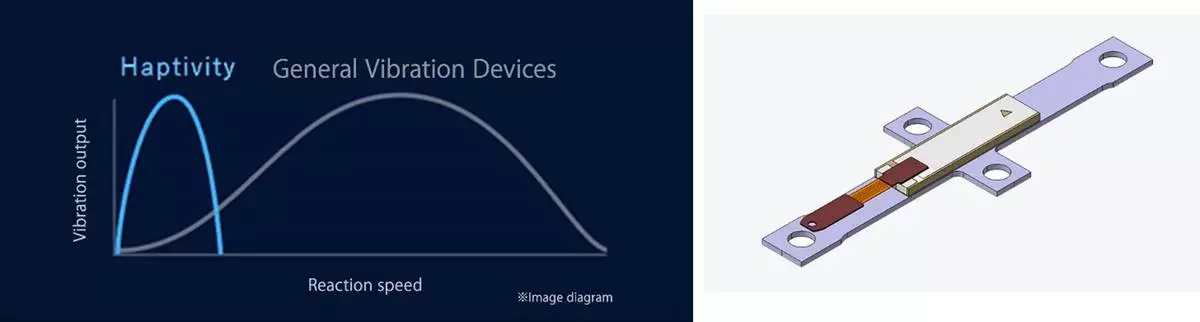
1. Excellent vibration response
HAPTIVITY® uses a piezoelectric ceramic vibrating element with excellent touch-response, delivering physical feedback that instantly confirms user input and eliminates duplicate commands.
2. Realistic tactile feeling
A mechanical button delivers two sensations: on press, and on release. Conventional tactile technology generates a single sensation (when pressed), which differs from the tactile feeling of a button. In contrast, HAPTIVITY® generates an initial vibration when pressed, and a second vibration when released, providing a more realistic button sensation.
3. Tactile confirmation improves operation
Immediate tactile feedback confirms user input instantly, ensuring reliable operation and eliminating ambiguity. This makes HAPTIVITY® ideal for demanding applications, including mobility, telemedicine, and industrial equipment.
4. Recessed buttons save space, increasing design freedom
HAPTIVITY® facilitates compact, thin, flat designs, allowing recessed buttons that promote space-saving and greater freedom of design.
Details of "HAPTIVITY"
https://europe.kyocera.com/products/lcds_glass_glass_touch_panels/prd/haptivity/index.html
About Kyocera
Kyocera Corporation (TOKYO:6971, https://global.kyocera.com/ ), the parent and global headquarters of the Kyocera Group, was founded in 1959 as a producer of fine ceramics (also known as “advanced ceramics”). By combining these engineered materials with metals and integrating them with other technologies, Kyocera has become a leading supplier of industrial and automotive components, semiconductor packages, electronic devices, smart energy systems, printers, copiers, and mobile phones. During the year ended March 31, 2024, the company’s consolidated sales revenue totaled 2 trillion yen (approx. US$13.3 billion). Kyocera is ranked #874 on Forbes magazine’s 2024 “Global 2000” list of the world’s largest publicly traded companies, and has been named among “The World’s 100 Most Sustainably Managed Companies” by The Wall Street Journal.
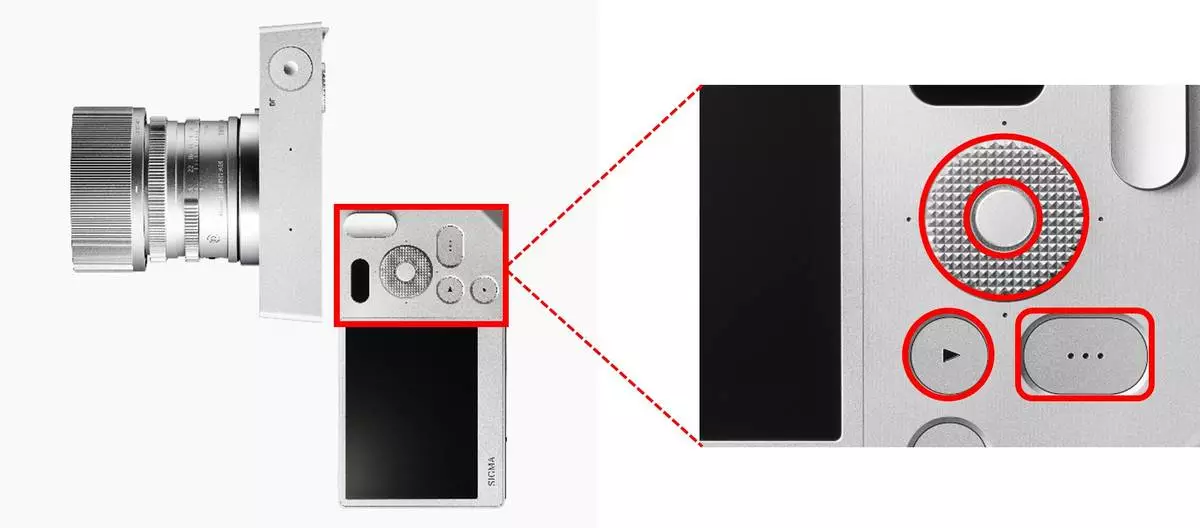
Kyocera Integrates HAPTIVITY® Technology into Sigma BF Mirrorless Camera
Excellent vibration response

“Sigma BF” full-size mirrorless camera with recessed buttons incorporating Kyocera’s HAPTIVITY® technology (image courtesy of Sigma)
President Donald Trump’s administration released a lengthy review of transgender health care on Thursday that advocates for a greater reliance on behavioral therapy rather than broad gender-affirming medical care for youths with gender dysphoria.
The 409-page Health and Human Services report questions standards for the treatment of transgender youth issued by the World Professional Association for Transgender Health and is likely to be used to bolster the government’s abrupt shift in how to care for a subset of the population that has become a political lightning rod. Medical experts sharply criticized it as inaccurate.
This new “best practices” report is in response to an executive order Trump issued days into his second term that says the federal government must not support gender transitions for anyone under age 19.
“Our duty is to protect our nation’s children — not expose them to unproven and irreversible medical interventions,” National Institutes of Health Director Dr. Jay Bhattacharya said in a statement. “We must follow the gold standard of science, not activist agendas.”
The report also questions the ethics of medical interventions for transgender young people, suggesting that adolescents are too young to give consent to life-changing treatments that could result in future infertility.
It also cites and echoes a report in England last year that questioned medical approaches to gender dysphoria as England's health services stopped prescribing puberty blockers to such children outside of research settings.
Child and adolescent psychiatrist Dr. Scott Leibowitz, a co-author of the WPATH standards for youth, said the new report “legitimizes the harmful idea that providers should approach young people with the notion that alignment between sex and gender is preferred, instead of approaching the treatment frame in a neutral manner.”
The report contradicts American Medical Association guidance, which urges states not to ban gender-affirming care for minors, saying that “empirical evidence has demonstrated that trans and non-binary gender identities are normal variations of human identity and expression.”
It also was prepared without input from the American Academy of Pediatrics, according to its president, Dr. Susan Kressly.
“This report misrepresents the current medical consensus and fails to reflect the realities of pediatric care,” Kressly said. She said the AAP was not consulted “yet our policy and intentions behind our recommendations were cited throughout in inaccurate and misleading ways.”
HHS said its report does not address treatment for adults, is not clinical guidance and does not make any policy recommendations. However, it also says the review “is intended for policymakers, clinicians, therapists, medical organizations, and importantly, patients and their families,” and it declares that medical professionals involved in transgender care have failed their young patients.
Gender-affirming care for transgender youth under standards widely used in the U.S. includes supportive talk therapy and can — but does not always — involve puberty blockers or hormone treatment.
The Trump administration’s report says “many” U.S. adolescents who are transgender or are questioning their gender identity have received surgeries or medications. In fact, such treatments remain rare as a portion of the population. Fewer than 1 in 1,000 adolescents in the U.S. received gender-affirming medication — puberty blockers or hormones — according to a five-year study of those on commercial insurance released this year. About 1,200 patients underwent gender-affirming surgeries in one recent year, according to another study.
Many U.S. adolescents with gender dysphoria may decide not to proceed with medications or surgeries. Medical association recommendations say the best care includes developing a plan with medical experts and family members that includes psychotherapy for each young person.
“It’s very chilling to see the federal government injecting politics and ideology into medical science,” said Shannon Minter, the legal director at the National Center for Lesbian Rights. Minter said the report could create fear for families seeking care and for medical providers.
“It’s Orwellian. It is designed to confuse and disorient,” Minter added.
Jamie Bruesehoff, a New Jersey mom, said her 18-year-old daughter, who was assigned male at birth, identified with girls as soon as she could talk. She began using a female name and pronouns at eight and received puberty blockers at 11 before eventually beginning estrogen therapy.
“She is thriving by every definition of the word,” said Brusehoff, who wrote a book on parenting gender-diverse children. “All of that is because she had access to this support from her family and community and access to evidence-based gender-affirming health care when it was appropriate.”
Laura Hoge, a New Jersey therapist who treats young people with gender dysphoria said, “I've seen that pain lift when they receive the gender-affirming care they need. This report denies that truth. It trades their healing for politics.”
Dr. Jack Drescher, a New York psychiatrist and psychoanalyst who works on sexual orientation and gender identity issues, said the report is one-sided and “magnifies the risks of treatments while minimizing benefits of the treatments.”
A judge has blocked key parts of Trump’s order, which includes denying research and educational grants for medical schools, hospitals and other institutions that provide gender-affirming care to people 18 or younger. Several hospitals around the country ceased providing care. The White House said Monday that since Trump took office, HHS has eliminated 215 grants totaling $477 million for research or education on gender-affirming treatment.
Most Republican-controlled states have also adopted bans or restrictions on gender-affirming care. A U.S. Supreme Court ruling is pending after justices heard arguments in December in a case about whether states can enforce such laws.
The Jan. 28 executive order is among several administration policies aimed at denying the existence of transgender people. Trump also has ordered the government to identify people as either male or female rather than accept a concept of gender in which people fall along a spectrum, remove transgender service members from the military, and bar transgender women and girls from sports competitions that align with their gender. This month, HHS issued guidance to protect whistleblowers who report doctors or hospitals providing gender-affirming care. Judges are blocking enforcement of several of the policies.
This latest HHS report, which Trump called for while campaigning last year, represents a reversal in federal policy. The U.S. Substance Abuse and Mental Health Services Administration, which is part of HHS, found that no research had determined that behavioral health interventions could change someone’s gender identity or sexual orientation. The 2023 update to the 2015 finding is no longer on the agency’s website.
While Health Secretary Robert F. Kennedy Jr. has repeatedly pledged to practice “radical transparency,” his department did not release any information about who authored the study. The administration says the new report will go through a peer-review process and will only say who contributed to the report after “in order to help maintain the integrity of this process.”
The report says that medical groups have relied on medical treatment rather than behavioral therapy for transgender youth partly because of a “mischaracterization of such approaches as ‘conversion therapy,’” which about half the states have banned for minors.
The American Academy of Child & Adolescent Psychiatry has said that evidence shows conversion therapies inflict harm on young people, including elevated rates of suicidal ideation.

FILE - Health and Human Services Secretary Robert F. Kennedy Jr. speaks at the Rx and Illicit drug Summit, April 24, 2025, in Nashville, Tenn. (AP Photo/George Walker IV, File)

FILE - Children hold signs and transgender pride flags as supporters of transgender rights rally by the Supreme Court, Dec. 4, 2024, in Washington. (AP Photo/Jacquelyn Martin, File)

File - Supporters of transgender rights rally by the Supreme Court, Dec. 4, 2024, in Washington. (AP Photo/Jacquelyn Martin, File)

FILE - President Donald Trump holds up an executive order after signing it at an indoor Presidential Inauguration parade event in Washington, Jan. 20, 2025. (AP Photo/Matt Rourke, File)