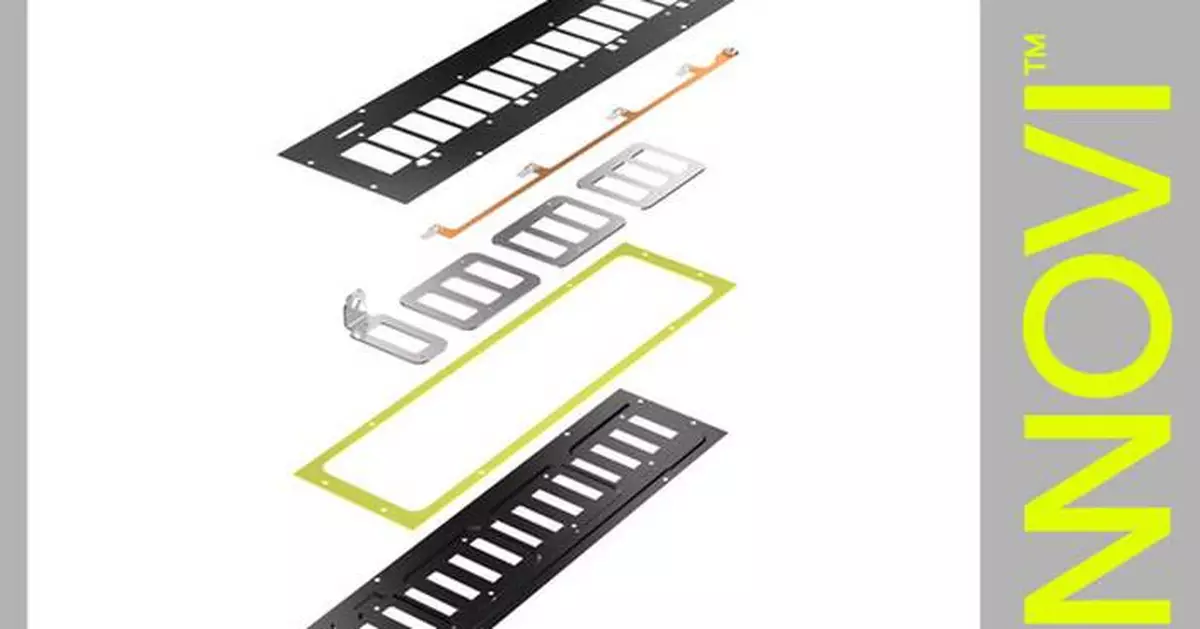
Vertically integrated production and precision processes help lower costs, simplify logistics and reduce development time
SINGAPORE, Oct. 7, 2024 /PRNewswire/ -- ENNOVI, a mobility electrification solutions partner, augments its prismatic and cylindrical battery cell contacting systems with the launch of ENNOVI-CellConnect-Pouch. Designed for the battery manufacturers favoring the pouch cell form factor, the new cell contacting system prioritizes cost-effectiveness with our FDC technologies, faster manufacturing cycle time, and aids the fast release of gasses produced during a thermal event.
"With the ENNOVI-CellConnect-Pouch cell contacting system we offer battery manufacturers a total vertically integrated production solution enabling us to produce a part at a lower cycle time using hot lamination," says Gustavo Cibrian, Product Manager, ENNOVI. "The system's design removes the need for traditional plastic cell carriers, made out of glass-reinforced plastic, and eliminates the carrier storage and molding lines, and heat staking process."
ENNOVI's advanced lamination technology ensures the structural integrity and durability of the battery pack. Utilizing flexible die-cut circuit (FDC) technology for the low voltage signal traces, instead of a conventional flexible printed circuit (FPC), reduces production time and costs while minimizing environmental impact.
The upper dielectric layer incorporates the FDC copper traces, while the lower layer is pre-cut to the shape of the current collectors. Both layers are made of automotive-grade PI or PET material with good electrical insulation and temperature stability properties. The two layers are laminated together with all built-in fuse traces or surface mount fuses and NTC temperature sensors layered in between, streamlining material usage and creating a thin design. The overall design also helps mitigate battery thermal runaway by achieving a fast exhaust release time.
For more information about ENNOVI-CellConnect-Pouch, please visit ennovi.com/cell-contacting-system-pouch.
About ENNOVI:
At ENNOVI we design and manufacture products and solutions for electrical battery platform developments, power components, and signal interconnect design solutions. Using our decades of experience in electro-mechanical engineering and high-precision manufacturing, we work with EV OEMs and suppliers to bring their ideas to life. As a Mobility Electrification Solutions Partner, we accelerate the process for our EV customers by offering complete end-to-end manufacturing capabilities from R&D, design, tooling to production. Learn more at www.ennovi.com.
Press Contacts:
Selvan Wilhelm
Selvan.Wilhelm@ennovi.com
Erin McMahon
erin.mcmahon@publitek.com
Publitek Germany
Alessandra Rosati
alessandra.rosati@publitek.com
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

ENNOVI Revolutionizes Pouch Cell Battery Design with the Introduction of ENNOVI-CellConnect-Pouch
KYOTO, Japan, Oct. 7, 2024 /PRNewswire/ -- Rege Nephro Co., Ltd. (https://www.regenephro.co.jp/) is excited to announce that the company has successfully developed a funding plan of total 25 billion yen in new Series B funding and raised 22.5 billion yen with the first payment. This significant investment brings the company's total funding to 44 billion yen. The funding round attracted a mix of strategic investors, including DCI Partners Co., Ltd. as a lead investor, JIC Venture Growth Investments Co., Ltd., Nippon Venture Capital CO., Ltd., TOHOKU University Venture Partners Co., Ltd., Golden Asia Fund Ventures Ltd., Mitsui Chemicals, Inc., Global Brain Corporation, Kyoto University Innovation Capital Co., Ltd., JAFCO Group Co., Ltd., Mitsubishi UFJ Capital Co., Ltd., ASAHI KASEI CORPORATION, QB Capital, LLC who recognize the potential of Rege Nephro in delivering new treatments to patients suffering from their diseases. The newly acquired funds will be strategically used to advance the clinical trials of RN-014, our promising treatment in Phase II trials for Autosomal Dominant Polycystic Kidney Disease (ADPKD), and to prepare for clinical trials in the United States. Additionally, the funding will support the completion of preclinical trials for our second innovative product, RN-032, aimed at treating Chronic Kidney Disease (CKD) through cell therapy.
Investor Statements
DCI Partners Co., Ltd.
"We are excited to announce that we are the lead investor in this round of investment from our fund. We are fully committed to supporting the growth of Rege Nephro."
JIC Venture Growth Investments Co., Ltd.
"We strongly believe that his advanced drug discovery research based on Professor Osafune's discoveries will provide new therapeutic solutions for intractable renal diseases."
Kyoto University Innovation Capital Co., Ltd.
"We are delighted that Rege Nephro, which we have supported since before the company was founded, has finally entered the clinical stage. We hope that this funding will accelerate the clinical trials and bring a new therapeutic drug to patients as soon as possible."
JAFCO Group Co., Ltd.
"We hope that this financing will further accelerate Rege Nephro research and development in iPS drug discovery and provide new treatment options for patients suffering from diseases with limited treatment options."
Mitsubishi UFJ Capital Co., Ltd.
"Our continued investment reflects our confidence in Rege Nephro's rapid progress and the potential of Professor Osafune's iPS cell technology to significantly impact medical practice."
Series B investors
DCI Partners Co., Ltd.
JIC Venture Growth Investments Co.,
Nippon Venture Capital CO., Ltd
TOHOKU University Venture Partners Co., Ltd.
Golden Asia Fund Ventures Ltd.
Mitsui Chemicals, Inc.
Global Brain Corporation
Kyoto University Innovation Capital Co., Ltd.
JAFCO Group Co., Ltd.
Mitsubishi UFJ Capital Co., Ltd.
QB Capital, LLC
Industrial Technology Investment
SHIMADZU CORPORATION
SCREEN Holdings Co., Ltd.
Chushin Venture Capital Co.,Ltd.
SENSHU IKEDA CAPITAL CO.,LTD.
Toyo Seikan Group Holdings, Ltd.
ASAHI KASEI CORPORATION
i-Lab CVC1 Limited Liability Partnership
Others
About Rege Nephro Co., Ltd.
Rege Nephro Co., Ltd. is a clinical-stage biotech company utilizing induced pluripotent stem cell (iPSC)-based technology from the Center for iPS Cell Research and Application (CiRA), Kyoto University. It is developing therapeutics and cell therapy for kidney, liver, and pancreas diseases.
About RN-014
Tamibarotene, under development for ADPKD, is a retinoic acid receptor (RAR) agonist expected to potently inhibit cyst formation and improve renal function. The Phase II clinical trial began in December 2023. This trial includes a pharmacokinetic (PK) phase and a randomization phase. Various safety measures have been established, including phased patient enrollment, periodic independent safety monitoring, strict criteria for dose reduction and discontinuation of the investigational drug, and criteria for discontinuation of the entire trial. There have been no significant issues at this time.
About RN-032
Cell therapy using NPCs for Chronic Kidney Disease (CKD). Nephron progenitor cells (NPCs) are cells that give rise to nephrons. Rege Nephro has been tried implanting iNPCs (NPCs induced from allogeneic human iPSCs) into the damaged kidneys, and the improvements in renal function have been confirmed in the several mouse experiments.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Rege Nephro Co., Ltd. Successfully Completes Series B Funding to Accelerate Kidney Disease Therapy Trials