SEONGNAM, South Korea, Dec. 12, 2024 /PRNewswire/ -- SK bioscience, a global innovative vaccine and biotech company committed to promoting human health from prevention to cure, announced today that it has received approval from the Human Research Ethics Committee (HREC) in Australia for the clinical trial protocols of a Phase 1/2 trials for its mRNA-based Japanese encephalitis vaccine candidate, GBP560.
SK bioscience is establishing its mRNA vaccine platform through the development of vaccines against Japanese encephalitis virus as well as Lassa fever virus.
The Japanese Encephalitis Virus (viral family: Flaviviruses) and Lassa fever vaccines (viral family: Arenaviruses) will serve as prototype vaccines that could potentially be adapted in a matter of weeks for use against a future Disease X that might emerge from those same viral families. This approach is key to the 100 Days Mission, a global goal to accelerate the development of vaccines against pandemic threats to just over three months.
This joint project is based on an agreement with the Coalition for Epidemic Preparedness Innovations (CEPI), which committed up to USD 40 million initial funding (approximately KRW 57.4 billion) in funding in 2022 to support preclinical and early clinical trials. A further USD 100 million in funding could be made available at a later date to support late-stage trials/licensure to further validate the mRNA platform and have it ready for use in outbreak situations.
The partnership builds upon SK bioscience's expertise in cutting-edge vaccine platforms such as cell culture, bacterial culture, and recombinant technology. By expanding the company's capabilities to include the mRNA platform, the collaboration aims to be ready to rapidly respond to Disease X and contribute to equitable access to vaccines, including through a contractual commitment to, if successful, prioritize supply for low-and middle-income countries, produce vaccine volumes required to meet public health needs, and ensure affordable pricing.
The mRNA vaccine platform technology, which was first commercialized in the COVID-19 vaccines during the pandemic, is available for rapid mass production compared to traditional platforms by utilizing genetic sequences. This capability makes mRNA technology well-suited for pandemic response. Furthermore, the mRNA vaccine platform shows significant potential to be applied to the development of therapeutic drugs, signaling rapid market expansion.
According to global market research firm 'Nova One Advisor', the global mRNA therapeutics market is projected to surpass around USD 58.90 billion (approximately KRW 84.5 trillion) by 2033, registering a CAGR of 17.06% by 2033.
The company will evaluate the immunogenicity and safety of GBP560 in 402 healthy adult participants in Australia. In stage 1, the participants will receive two shots of low, medium, high-dose vaccine, or a control vaccine administered 28 days apart. Based on the stage 1 results, the optimal dosage and regimen will be determined for further assessment of the immunogenicity and safety compared to the control group in stage 2.
During the Phase 1/2 trials, SK bioscience plans to confirm neutralizing antibody titers and immune responses, along with collecting data on major adverse events, safety indicators, and the rate of adverse reactions. The company aims to secure interim results by 2026.
Previously, the company conducted preclinical studies beginning in December 2023, where the vaccine candidate demonstrated superior safety and immunogenicity through repeated-dose toxicity studies, safety pharmacology tests, animal efficacy trials, and protective immunity tests.
Dr. Kent Kester, Executive Director of Vaccine Research and Development at CEPI said, "mRNA technology is at the heart of CEPI's work to build a library of vaccine candidates that could be swiftly adapted next time Disease X emerges. Our work with SK bioscience aims to validate the rapid response mRNA platform against viruses from two of the viral families with the potential to spawn future epidemics and pandemics, contributing to the 100 Days Mission and to building a safer world for all."
Jaeyong Ahn, CEO of SK bioscience, said, "The mRNA vaccine platform, with its rapid development cycle, is a critical tool for pandemic preparedness. By leveraging our strong network of various partners, including international organizations, global companies, and the Korean government, SK bioscience is committed to successfully establishing the mRNA technology platform and contributing to global health, equitable access to vaccines and vaccine sovereignty."
CEPI also supported the development of SK bioscience's COVID-19 vaccine and is funding an ongoing project to support a broadly protective coronavirus vaccine.
About SK bioscience
SK bioscience is an innovative vaccine and biotech company, committed to vaccine development and manufacturing to enable more equitable access to vaccines around the world. Leveraging strengths on cutting-edge technologies, SK bioscience has been dedicated to promoting human health from prevention to cure across the globe. With the cooperation of domestic and international governments, regulatory agencies, healthcare providers, doctors, and medical experts, all of the SK colleagues are passionately committed to providing high-quality vaccines to those who need them and better public healthcare solutions.
- SK bioscience Website
- SK bioscience Linkedin
Contact
Communications Team
Changhyun Jin (jin99@sk.com), Jeannie S. Pak (j.pak@sk.com)
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

SK bioscience Receives Approval for Global Clinical Trials of mRNA Japanese Encephalitis Vaccine Candidate
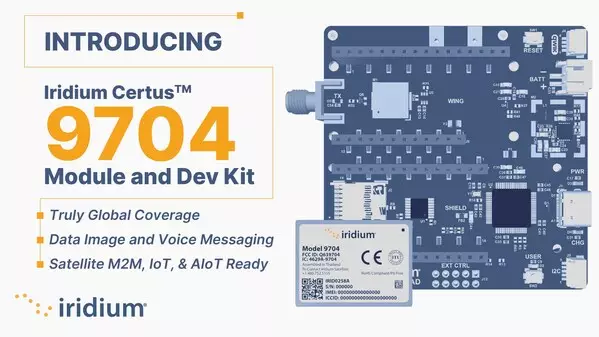
The new module is supported by the Iridium Certus 9704 Development Kit, and is ideal for data, audio messages and image transfers, while positioning companies as more future ready in anticipation of Satellite AIoT
MCLEAN, Va., Dec. 12, 2024 /PRNewswire/ -- Iridium Communications Inc. (NASDAQ: IRDM), a leading provider of global voice and data satellite communications, today launched the Iridium Certusâ„¢9704, its newest IoT module, and Iridium Certus 9704 Development Kit. This transformational new module, featuring Iridium Messaging Transport® (IMT®) technology, is the smallest and most powerful created by Iridium, ideal for supporting satellite IoT applications that require real-time data analysis, analytics and automated decision-making. The Iridium Certus 9704 provides larger file transfer sizes and faster message speeds than previous Iridium® IoT modules, delivering data, picture, and audio messages for industrial (IIoT), machine-to-machine (M2M) and remote personnel use cases. Like other Iridium Connected® devices, the Iridium Certus 9704 can provide two-way IoT services anywhere in the world, making fast and reliable connections to a broad array of applications.
The Iridium Certus 9704 architecture simplifies data transfer requirements for use cases like predictive maintenance, diagnostics, telemetry monitoring, remote asset tracking, and command and control for uncrewed aircraft, vehicles and vessels. The module's larger-sized data messages are also ideal for remote sensing applications protecting wildlife, detecting wildfires and flooding, and enable a new generation of purpose-built satellite messengers and personal safety devices.
"We've now packed more capability and value into the smallest module ever from Iridium," said Matt Desch, CEO, Iridium. "When companies choose to develop with Iridium, they know they are partnering with the leader in satellite IoT applications, and we'll support them through the development and deployment of their solutions. We're excited to see how they implement this new technology."
Designed for ease of integration, the Iridium Certus 9704 is 34% smaller than the Iridium 9603, 79% smaller than the Iridium 9602, and has an 83% reduction in idle power consumption compared to both. This power optimization makes the new module ideal to support battery powered applications, while taking advantage of two-way messaging, including delivery confirmation, over Iridium's low-latency, truly global satellite network.
Ideal for supporting traditional satellite IoT applications, the new module can also help organizations become more future ready as Artificial Intelligence (AI) becomes integrated with IoT services, known as AIoT (Artificial Intelligence of Things). With this integration, satellite AIoT services should quickly follow, and companies that deploy products with Iridium Certus 9704 modules inside can then choose to offload more computing to the cloud in a single message, where an AIoT engine can quickly make decisions and send new, actionable instructions back to the remote device. This can lessen the required edge device processing power, lower hardware cost, and increase battery life and overall device lifespan. With IMT at its core, a built-in topic-sorting capability means messages can be efficiently organized for delivery to the appropriate engine for various types of real-time data, audio or image analysis.
"The Iridium Certus 9704 module and Development Kit were designed with the developer in mind," said Omar Azad, associate director, product management, Iridium. "We want to make it as easy as possible for anyone to experience the new module's capabilities and quality of the IMT service. When they're ready to take the next step and build a product to take to market, we're there to support them with our decades of experience in the satellite IoT space."
The Iridium Certus 9704 Development Kit is a complete test kit featuring a motherboard, power supply, antenna, module and software from the popular Arduino platform. It was designed to provide maximum autonomy and flexibility for developers, coming with 1,000 free messages and GitLab hosted reference materials. The kit is initially available to prospective Iridium customers for evaluation of the Iridium Certus 9704 module and IMT data service. Iridium has partnered with Blynk to give developers an all-in-one platform for data visualization and a rapid unboxing-to-live-testing experience.
Iridium operates the world's only truly global, weather resilient satellite constellation designed to reliably deliver critical data to and from anywhere on the planet. As the satellite IoT market leader, Iridium's IoT service revenue has been growing at an 11% CAGR over the last 10 years. Iridium goes to market through more than 500 companies around the world that integrate its core technology modules, like the Iridium Certus 9704, and sell airtime access to its network.
The Iridium Certus 9704 has received FCC and ISED authorizations for use, with additional regulatory approvals pending.
For more information about Iridium, visit: www.iridium.com
About Iridium Communications Inc.
Iridium® is the only mobile voice and data satellite communications network that spans the entire globe. Iridium enables connections between people, organizations, and assets to and from anywhere, in real time. Together with its ecosystem of partner companies, Iridium delivers an innovative and rich portfolio of reliable solutions for markets that require truly global communications. In 2024, Iridium acquired Satelles and announced the Iridium Satellite Time and Location service. Iridium Communications Inc. is headquartered in McLean, Va., U.S.A., and its common stock trades on the Nasdaq Global Select Market under the ticker symbol IRDM. For more information about Iridium products, services, and partner solutions, visit www.iridium.com.
Forward-Looking Statements Disclosure
Statements in this press release that are not purely historical facts may constitute forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995. The Company has based these statements on its current expectations and the information currently available to us. Forward-looking statements in this press release include statements regarding the capabilities, benefits and availability of the Iridium Certus 9704 module and Development Kit. Forward-looking statements can be identified by the words "anticipates," "may," "can," "believes," "expects," "projects," "intends," "likely," "will," "to be" and other expressions that are predictions or indicate future events, trends or prospects. These forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements of Iridium to differ materially from any future results, performance or achievements expressed or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to, uncertainties regarding the company's ability to maintain the health, capacity and content of its satellite constellation, as well as general industry and economic conditions, and competitive, legal, governmental and technological factors. Other factors that could cause actual results to differ materially from those indicated by the forward-looking statements include those factors listed under the caption "Risk Factors" in the Company's Form 10-K for the year ended December 31, 2023, filed with the Securities and Exchange Commission (the "SEC") on February 15, 2024, and the Company's Form 10-Q for the quarter ended September 30, 2024, filed with the SEC on October 17, 2024, as well as other filings Iridium makes with the SEC from time to time. There is no assurance that Iridium's expectations will be realized. If one or more of these risks or uncertainties materialize, or if Iridium's underlying assumptions prove incorrect, actual results may vary materially from those expected, estimated or projected. Iridium's forward-looking statements speak only as of the date of this press release, and Iridium undertakes no obligation to update forward-looking statements.
Press Contact:
Investor Contact:
Jordan Hassin
Kenneth Levy
Iridium Communications Inc.
Iridium Communications Inc.
Jordan.Hassin@Iridium.com
Ken.Levy@Iridium.com
+1 (703) 287-7421
+1 (703) 287-7570
X: @Iridiumcomm
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Iridium Transforms Satellite IoT with the Launch of Revolutionary Iridium Certus 9704 Module











