SINGAPORE, Jan. 7, 2025 /PRNewswire/ -- BIPO, a leading global HR technology and services provider, has been recognised as a Major Contender in the Everest Group Multi-Country Payroll (MCP) Solutions PEAK Matrix® Assessment 2024. This marks the third consecutive year BIPO has secured this prestigious acknowledgement, reinforcing its commitment to payroll technology and service excellence.
Headquartered in Singapore and backed by four R&D centres across Asia, BIPO stands as a trusted partner for organisations navigating the complexities of the diverse and fast-evolving Asia Pacific region. With an in-depth understanding of APAC's regulatory landscapes and cultural nuances, BIPO delivers tailored HR and payroll solutions that empower businesses to stay agile and competitive. Its flagship HR Management System seamlessly integrates HR and payroll functions, providing insights that enhance workforce productivity and ensure seamless compliance.
Priyanka Mitra, Vice President at Everest Group, commented on BIPO's performance: "BIPO's commitment to offering an integrated and comprehensive HR ecosystem, along with its focus on expanding its geographic footprint, has reinforced its position as a major contender in the Multi-Country Payroll (MCP) Solutions PEAK Matrix® Assessment 2024. Its emphasis on enhancing stakeholder experience, demonstrated through ongoing investments in building a stronger sales partner network, onboarding strategic partners, and exploring opportunities in artificial intelligence (AI), machine learning (ML), and automation to enhance product capabilities, positions it well in the payroll market."
With the European HR tech market projected to grow to USD 88.38 billion by 2033, businesses are increasingly adopting automation and payroll digitisation to enhance employee experience and engagement. Platforms like BIPO's are pivotal in addressing these global demands, offering scalable, reliable, and compliant solutions that help organisations navigate the challenges of payroll and workforce management across borders.
To meet the rising adoption, BIPO has strategically expanded its footprint in Europe, establishing offices in Spain, Germany, the Netherlands, Turkey, and the UK. These additions bolster its global presence, which spans more than 40 offices worldwide including major regions like Latin America, and position BIPO as a trusted partner for multinational organisations seeking a comprehensive HR and payroll solution.
"This recognition from Everest Group validates our vision to become the leading Asia-based global HR tech and service provider," said Michael Chen, CEO of BIPO. "Our expanding global footprint, combined with our deep expertise in Asia-Pacific markets, uniquely positions us to serve multinational organisations that require seamless, compliant HR operations across diverse regions. We are focused on bridging the technological gap across borders, enabling businesses to operate with greater efficiency and insight."
BIPO's comprehensive HR solutions now serve businesses in over 160 global markets, delivering essential services such as Human Capital Management solutions, Global Payroll Outsourcing, and Employer of Record services through its award-winning HR Management System.
Learn more at www.biposervice.com or follow BIPO on LinkedIn.
About BIPO
Established in 2010 and headquartered in Singapore, BIPO is a trusted provider of payroll and people solutions in over 160 global markets. Our comprehensive HR offerings include Human Capital Management solutions, Global Payroll Outsourcing, and Employer of Record services, powered by our award-winning HR Management System and Athena BI (Business Intelligence) platform. At BIPO, we deliver customised services and scalable tech-enabled solutions that automate processes, simplify workflows, and generate actionable insights.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

BIPO Strengthens Global HR Tech Leadership with Third Recognition in Everest Group's PEAK Matrix® Assessment

BIPO Strengthens Global HR Tech Leadership with Third Recognition in Everest Group's PEAK Matrix® Assessment
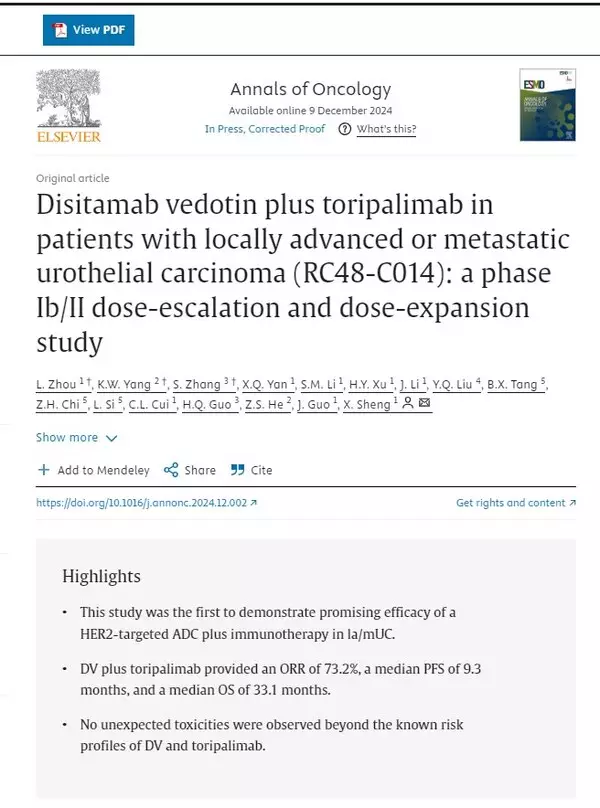
YANTAI, China, Jan. 8, 2025 /PRNewswire/ -- On Janurary 7th, 2025, Annals of Oncology (IF: 56.7), a top oncology journal globally, published remarkable long-term follow-up results of a phase 1b/2 clinical trial on Disitamab Vedotin (DV) (developed by Remegen Co., Ltd) combined with Toripalimab in treating locally advanced or metastatic urothelial carcinoma (la/mUC) (NCT04264936, study ID: RC48-C014). This trial was supervised by Professor Jun Guo and Professor Xi'nan Sheng's teams from Peking University Cancer Hospital.
It is the first time that long-term follow-up data has been released for a HER2-targeted antibody-drug conjugate (ADC) and PD-1 inhibitor combination therapy in treating la/mUC, marking it a significant milestone. The nearly-three-year follow-up data revealed an objective response rate (ORR) of 73.2% and median overall survival (OS) of 33.1 months, superior to data published from any other prospective clinical studies on ADC plus PD-1 combination therapies for la/mUC.
New Treatment Options for Patients with La/mUC
UC is the sixth most common cancer worldwide. GLOBOCAN 2022 estimated the year 2021 saw 614,298 new cases and 220,596 deaths of UC. In recent years, the prognosis for patients with la/mUC has significantly improved with new drugs and combination therapies approved, among which ADCs demonstrated outstanding potential.
As a HER2-targeted ADC, DV has been approved in China for patients with HER2-overexpressing (defined as immunohistochemistry [IHC] test results of 2+ or 3+) la/mUC previously treated with platinum-containing chemotherapy. The approval is based on the pooled results of two studies (NCT03507166 and NCT03809013, study IDs: RC48-C005 and RC48-C009) where the ORR registered 50.5% and the median duration of response (DOR) registered 7.3 months.
Multiple clinical studies on la/mUCin recent years have confirmed the synergistic antitumor effects of ADC combined with immunotherapy. NCT04264936 offered stronger evidence as its long-term follow-up results published in the Annals of Oncology demonstrated the high response rate, significant survival benefits, and manageable safety profile of the DV and Toripalimab combination therapy.
DV Combined with Toripalimab: High Response Rate and Prolonged Survival
NCT04264936 is an open-label, multicenter, investigator-initiated phase 1b/2 clinical trial investigating the safety and efficacy of DV in combination with Toripalimab for the treatment of patients with HER2-expressing la/mUC. The dose-escalation study (phase 1b) assessed two dose levels of DV (1.5 and 2.0 mg/kg) combined with Toripalimab (3.0 mg/kg) to determine the recommended phase 2 dose which was then evaluated in the dose-expansion stage (phase 2).
From August 2020 to December 2021, 41 patients were enrolled with a median age of 66 years. 53.7% of the participants were male, 70.7% had an ECOG performance status score of 1, 17 (41.5%) had lung metastasis, and 10 (24.4%) had liver metastasis.
As of March 1, 2024, among all participants, the ORR was 73.2% with 4 (9.8%) achieving complete response and 26 (63.4%) achieving partial response, the DCR was 90.2%, the median progression-free survival (PFS) was 9.3 months, the median DOR was 8.6 months, the median OS was 33.1 months and the 36-month OS rate was 49.2%.
Subgroup analysis revealed ORR benefits across all subgroups regardless of the number of prior lines of systemic treatments, HER2 expression (IHC 1+/2+/3+), and PD-L1 expression status. The ORRs for chemotherapy-naïve patients and those who progressed on platinum-based chemotherapy were 76.0% (19/25) and 68.8% (11/16), respectively. The ORRs for patients with HER2 IHC 3+, 2+, 1+, or 0 were 80.0%, 84.2%, 64.3%, and 33.3%, respectively. Compared with the three HER2 IHC 0 participants (one of whom achieved partial response), those with HER2 expression (IHC 1+/2+/3+) had a higher ORR (76.3% vs 33.3%) and longer PFS (median PFS: 9.3 vs 1.7 months).
In summary, the DV and Toripalimab combination therapy has preliminarily demonstrated promising efficacy and manageable safety profile among patients with la/mUC, wherein those with HER2 expression (IHC 1+/2+/3+) achieved high response rates and long-term survival benefits. These findings support the further exploration and validation of the benefits of DV combined with PD-1 inhibitors in treating la/mUC.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Published in Annals of Oncology: Disitamab Vedotin Combined with PD-1 Inhibitor is a Promising Treatment for Locally Advanced or Metastatic Urothelial Carcinoma












