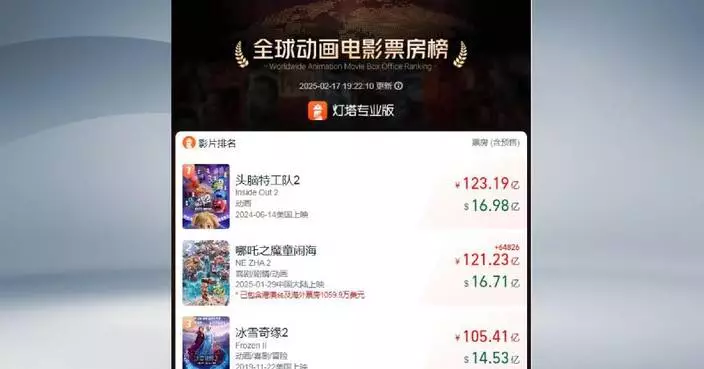
A groundbreaking new lung cancer drug called Ivonescimab, developed by a Chinese company, is making waves in the global biotech industry and closing the gap with leading pharmaceutical companies in cancer drug development.
"This is our company's newly developed drug, Ivonescimab. In a randomized, double-blind phase three clinical trial for lung cancer, it surpassed Keytruda, the leading drug in the field," said the drug maker Akeso's chairman and CEO Dr Michelle Xia.
According to the World Conference on Lung Cancer, patients can live on Ivonescimab for 11.1 months before disease progression, compared to only 5.8 months on Keytruda, the world's top-selling cancer medication, which has generated over 130 billion U.S. dollars in sales for its American manufacturer.
"For the pharmaceutical industry, developing a drug that surpasses Keytruda and benefits more patients is a remarkable achievement. Many companies worldwide are striving to develop such drugs, but all of their phase three trials have failed. Our phase three clinical trial is the first in the world to achieve a strongly positive result in a randomized, double-blind study," said Dr Xia.
Akeso spent a decade developing Ivonescimab. Dr. Xia said world-class scientists were brought together to achieve this result, while the company also benefited from the support of the Chinese government.
"We are fortunate that, in the early stages of our company being established, the government began prioritizing the biopharmaceutical industry. Both central and local authorities have invested substantial human and material resources to support the industry's development," she said.
The breakthrough of Ivonescimab might align with a larger trend in China. In recent years, China has made significant strides in pharmaceutical innovation, with an increasing number of new drugs being developed and added to the pipeline.
"A key indicator we use is the number of research and development pipelines. This metric not only reflects our past investment in research and development but, more importantly, serves as a projection of our research and development potential. Based on this indicator, China currently ranks second in the world, just behind the United States," said Wang Shan, associate professor at the Business School of Sun Yat-sen University.
Professor Wang highlighted China’s advantages in pharmaceutical innovation, emphasizing its well-established supply chain, lower research and development costs, and abundant clinical resources.
"China has a well-established pharmaceutical supply chain. Additionally, our research and development costs are relatively low, thanks to abundant clinical resources and a large patient population," she said.
While recognizing these strengths, she also acknowledged that a gap remained between China and the U.S. in research and development pipelines, with China's numbers at about half of America's. However, she emphasized the significant potential for growth and the increasing drive for innovation.
New Chinese cancer drug outperforms global leader, draws international attention