TIANJIN, China, March 18, 2025 /PRNewswire/ -- On March 12, 2025, China's Center for Drug Evaluation (CDE) approved Tasly's Investigational New Drug (IND) application for NR-20201, an allogeneic adipose-derived mesenchymal stromal cell, sparking discussions about the historical development of mesenchymal stromal cell drugs.
In a groundbreaking development, Ryoncil, the first mesenchymal stromal cell (MSC) drug approved by the U.S. FDA, has made headlines not only for its innovative treatment approach but also for its off-the-charts pricing: a single-infusion costing $194,000, and the total cost for a complete treatment regimen of 8 infusions staggering at $1.55 million – February 26, 2025.
1. Confounded Identity of MSCs under the "Stem Cell" Overshadowing
In 1991, Case Western Reserve University Biologist Arnold Caplan, based on his scientific studies and pre-clinical experimentation, coined the term "mesenchymal stem cells or MSCs" [1] devoted to a special cell population found in mesenchymal tissues and capable of division and differentiation, presenting a high potential for regenerative tissue repair [1,2].
In 1992, following the identification of MSCs, Osiris Pharmaceuticals was founded, devoting its efforts to developing MSC-based drugs.
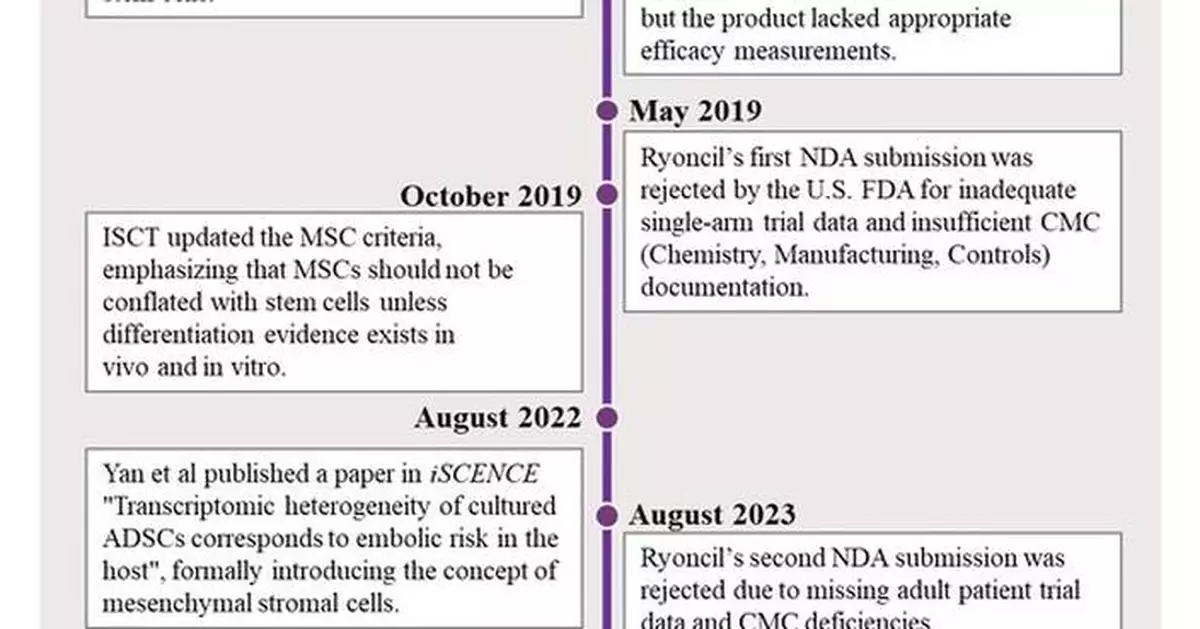
In 2012, the U.S. FDA rejected Osiris' bone marrow-derived Prochymal, declaring its purported stem cell regeneration mechanisms "unsupported by clinical evidence" - a verdict documented in both SEC filings (Osiris 10-K) and later codified in the U.S. FDA's 2017 Cell Therapy Guidelines. The failure reflected critical drawbacks: the lack of validated potency assays, undefined mechanisms of action, and unproved "stem cell" differentiation in vivo.
In 2013, Mesoblast acquired Prochymal and renamed it as Ryoncil (Remestemcel-L) - a name retaining the contested "stem" descriptor that would continue the same trail for the regulatory greenlight journey.
May 2019, Ryoncil's first New Drug Application (NDA) sparked an immediate pushback by the U.S. FDA, with a clearly stated rationale including unreliable single-arm trial results and critical drawbacks in chemistry, manufacturing, and controls (CMC).
March 2023, a second NDA submission of the same product by Mesoblast encountered another rejection for lacking controlled trials with adult patients and ongoing CMC issues.
These repeated failures reveal a significant flaw in the pharmaceutical landscape of MSCs. Ryoncil, while labeled as a mesenchymal stem cell, had not convincingly demonstrated its differentiation capacity as the mechanism of action for tissue repair. In contrast, experimental and clinical data indicated that the therapeutic efficacy of MSCs was mediated through the microenvironment via excreta secreted by MSCs after their homing to the target tissue. Thus, the industry confronted a lack of standardized protocols for a clear identification of MSCs. Because MSCs have been overshadowed by stem cells in their identity, the twist between an ambiguously understanding of the mechanism of action of MSCs and an inappropriate usage of MSCs as stem cells in clinical trials resulted in unpredictable risks and inconsistent efficacy.
2. Scientific Breakthroughs Revealed the Distinction between MSCs and Stem Cells
In 2016, Dr. Caplan, scrutinizing from his experimental studies and clinical observations, corrected his previous definition of MSCs as medicinal stem cells, and clarified that MSCs are stromal cells or so called "medicinal signaling cells". This redefinition proved crucial for framing MSCs therapeutic applications [3]. However, there were no specific markers to distinguish MSCs from stem cells, leading to a continuation of the mislabeling of MSCs as stem cells.
In 2022, Yan and colleagues from Tasly Stem Cell Biology Laboratory published a pivotal study in iScience "Transcriptomic heterogeneity of cultured ADSCs corresponds to embolic risk in the host". This study used most advanced technology of single-cell RNA sequencing in combination with machine learning to explicitly analyze transcriptomic profile at single cell level for the first time. The results demonstrated that MSCs are stromal cells, not stem cells and they are heterogeneous even derived from the same tissue of a single donor. The heterogeneous nature of MSCs constitutes the fundamental reason for unpredicted risks and inconsistent therapeutic efficacy [4].
February 2025, Yan et al published another definitive study on MSCs "Unveiling distinctions between mesenchymal stromal cells and stem cells by single-cell transcriptomic analysis" in Heliyon [5]. This is a landmark study. By utilizing single-cell RNA sequencing and pseudotime trajectory analysis, the team revealed the fundamental distinctions between MSCs and stem cells. These distinctions not only clearly distinguish MSCs from stem cells but also provide unambiguous biomarkers for the identification of MSCs, establishing the standard for MSC products. Consequently, it evidently cleans up the roadblock for the advancement of therapeutic aplantation of MSCs.
3. The Historical Turning Point of MSCs' Rollercoasting Journey
October 2024, the U.S. FDA greenlighted the first clinical trial for a well-defined mesenchymal stromal cell (MSC) therapy submitted by Tasly Stem Cell Research team, led by Dr. Kaijin Yan, marking a paradigm shift from confounded stem cell therapy to authentic MSC therapy [6]. This would trigger a series of chain reactions in cell therapy regulation.
December 2024, immediately following the approval of the world's first mesenchymal stromal cells clinical trial, the U.S. FDA granted Ryoncil market approval authorization as a mesenchymal stromal cell therapy - marking the world's first approved mesenchymal stromal cell product [7].
March 2025, China's Center for Drug Evaluation (CDE) approved the Investigational New Drug (IND) application of NR-20201[8], an allogeneic adipose-derived mesenchymal stromal cell injection independently developed by Tasly, for the country's first mesenchymal stromal cell drug, marking another significant milestone in the global advancement of MSC therapies.
The $1.55 Million Pricing Embedded 30 Years' Scientific Understanding
The off-the-charts pricing of Ryoncil shattered public perceptions of new drug market. Few, however, understand that the true cost encapsulates not just the bag of suspended cells but also the thirty-year journey of scientific understanding of MSCs, a substantially collective investment in innovative research and clinical translation.
The transition from "stem cells" to "stromal cells" signifies a profound reassessment of the role of MSCs in medicine. The extensive investments in research and development, combined with clinical trials stalled by unclear mechanisms and ongoing regulatory challenges, all contribute to Ryoncil's considerable pricing.
In this revolution, Ryoncil's price reflects not only the launch of the first mesenchymal stromal cell therapy but also the industry's hard-earned recognition of the scientific foundation that is fundamental to advanced cell therapy.
References
[1] Caplan A I. Mesenchymal stem cells. J Orthop Res. 1991 Sep;9(5):641-50.
[2] Caplan A I. Bone development and repair[J]. Bioessays, 1987, 6(4): 171-175.
[3] Caplan A I. MSCs: the sentinel and safe-guards of injury[J]. Journal of cellular physiology, 2016, 231(7): 1413-1416.
[4] Yan K, Zhang J, Yin W, Harding JN, Ma F, Wu D, Deng H, Han P, Li R, Peng H, Song X, Kang YJ. Transcriptomic heterogeneity of cultured ADSCs corresponds to embolic risk in the host. iScience. 2022 Aug 4;25(8):104822.
[5] Yan K, Ma F, Song X, Wang H, Liu P, Zhang J, Jin X, Han P, Zuo X, Kang YJ. Unveiling distinctions between mesenchymal stromal cells and stem cells by single-cell transcriptomic analysis. Heliyon. 2025 Feb 8;11(4):e42311.
[6] FDA Investigational New Drug Application: NO.30788
[7] FDA approves first mesenchymal stromal cell (Ryoncil) therapy to treat steroid refractory acute graft versus host
[8] Center for Drug Evaluation of the National Medical Products Administration, China: CXSL2400882.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
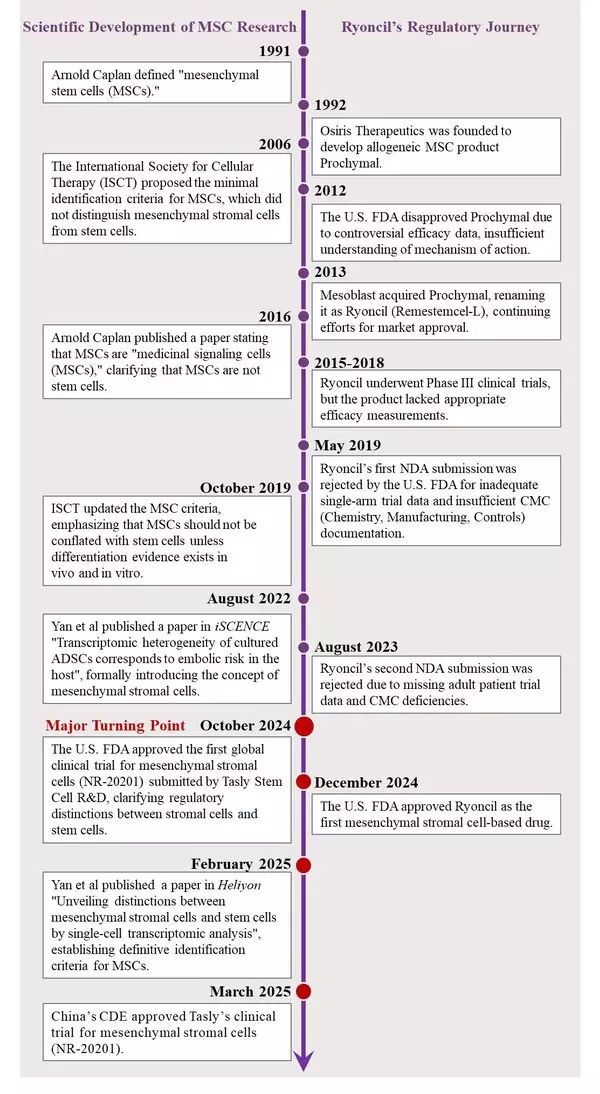
A chronographic analysis of MSC's rollercoasting journey