ONTARIO, Calif., July 31, 2024 /PRNewswire/ -- Leading augmented reality (AR) medical technology company SURGLASSES today announced that, in collaboration with exclusive distributor Goodlifeintertrade.co. Ltd., it has successfully registered and clinically implemented its AR-based Caduceus S surgical navigation system in Thailand. This groundbreaking system is designed to assist various minimally invasive surgeries, significantly enhancing surgical precision and patient outcomes.
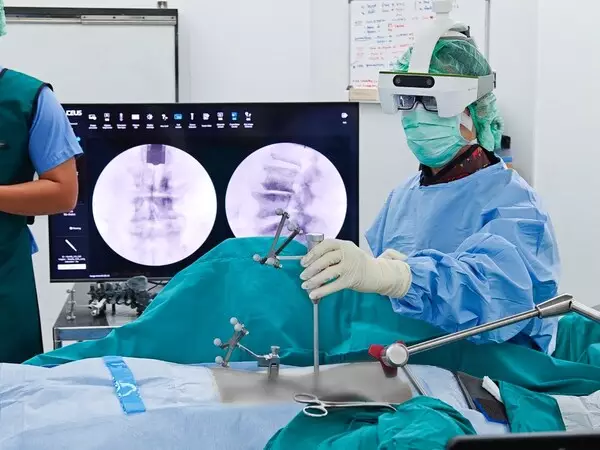
The first AR-guided surgery using only the C-arm in Thailand was successfully performed at Thailand Veterans General Hospital by Dr. Chaiyos Chaichankul. The 78-year-old patient had preoperative symptoms of leg numbness, radiating pain, and difficulty walking. After the surgery, the patient's nerve numbness in the legs disappeared, and the pain was significantly reduced. The day after the surgery, the patient could sit up and walk, and was discharged four days later, resuming work in two weeks. Dr. Chaiyos Chaichankul remarked, "The AR technology of the Caduceus S surgical navigation system allows us to precisely locate and treat the affected area, greatly increasing the success rate of surgeries and accelerating patient recovery."
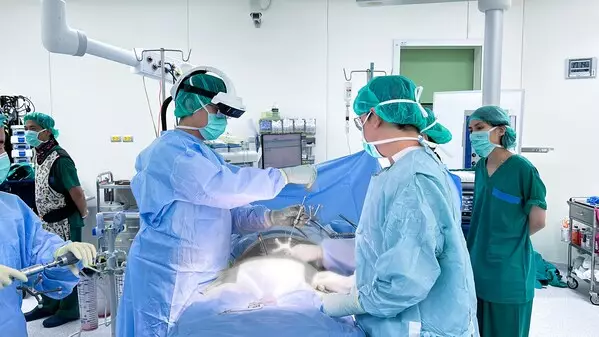
Additionally, SURGLASSES completed the first AR-guided surgery combined with O-arm technology at Chulalongkorn Hospital, led by renowned Associate Professor Prof. Wicharn Yingsakmongkol, M.D., and performed by Dr. Vit. The patient, a 48-year-old woman, had a preoperative back pain level of 10 out of 10. Post-surgery, her pain immediately reduced to 4, and two weeks later, it was completely gone. Dr. Vit stated, "Using the Caduceus S system in surgery not only enhances our precision but also shortens patient recovery time. We look forward to utilizing this innovative technology in more surgeries in the future."
The Caduceus S AR surgical navigation system is an advanced tool specifically designed for augmented reality spine surgery. Through its 3D visualization capabilities, surgeons can clearly view the internal anatomy of patients during procedures, including bones, tissues, and organs, thereby improving surgical accuracy and outcomes. AR minimally invasive spine surgery and spine navigation technology are becoming integral parts of modern healthcare, with SURGLASSES leading the charge in this field.
SURGLASSES CEO Min-Liang Wang commented, "We are thrilled with the significant clinical outcomes achieved by the Caduceus S surgical navigation system in Thailand. We are grateful for the collaboration with Dr. Chaiyos Chaichankul as well as the efforts of our research teams in Taiwan and the US. These successes demonstrate the potential of our technology to enhance the quality of healthcare."
SURGLASSES is dedicated to addressing the limitations of current medical imaging technology through the use of AR. The Caduceus S system enables clinicians to visualize patients' internal anatomical structures in 3D during surgery, including bones, tissues, and organs, thereby improving surgical precision and outcomes. The AR navigation technology also allows for future collaboration with remote clinicians through shared visualization and real-time communication, providing guidance during collaborative patient care.
SURGLASSES is an innovative medical technology company with subsidiaries in the US and Taiwan, committed to advancing human health through its AR digital augmented reality ecosystem. The Caduceus S augmented reality visualization platform aims to unlock the full potential of 3D data, transforming image-guided medical procedures through intuitive 3D X-ray visualization. The system has received FDA 510(k) clearance, as well as approvals from Taiwan TFDA and Thailand FDA, and is actively expanding its global market presence.
Through these advanced technologies, SURGLASSES is shaping the future of augmented reality surgical navigation systems, providing medical professionals worldwide with more efficient and precise surgical tools. The successful application of the Caduceus S marks a new era in medical technology, promising better surgical experiences and recovery outcomes for patients around the globe.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
SURGLASSES Announces Successful First Clinical Implementation of Caduceus S AR Surgical Navigation System in Thailand
SURGLASSES Announces Successful First Clinical Implementation of Caduceus S AR Surgical Navigation System in Thailand

SURGLASSES Announces Successful First Clinical Implementation of Caduceus S AR Surgical Navigation System in Thailand
By Greenyn Biotechnology Co., Ltd.
TAIPEI and TAICHUNG, Sept. 9, 2024 /PRNewswire/ -- Modern lifestyles characterized by irregular routines, high-fat and high-salt diets, and a preference for refined foods, coupled with busy schedules, high stress, overeating, and insufficient physical activity, have led to the emergence of the modern civilization ailment known as "metabolic syndrome."
Metabolic syndrome encompasses issues like abnormal blood lipids, blood sugar, and abdominal obesity (central obesity). Insulin resistance is the primary cause of metabolic syndrome. Prolonged poor blood sugar control can easily lead to diabetes and even other chronic diseases. According to statistics from Ministry of Health, Labour and Welfare, the number of diabetes patients in Japan has exceeded 10 million, with an increasing trend as the population ages. Driven by this substantial demand, bitter melon extract emerged, leading to their popularity in the market.
Potential Plant Insulin
Bitter melon (Momordica charantia Linn.), a member of the Cucurbitaceae family, is a traditional Chinese herbal medicine. Many studies have confirmed that bitter melon contains various blood sugar-lowering components, primarily including terpenes, phytosterols, and peptides. There are many related products on the market extracted from bitter melon (see Table 1). The mechanisms of action range from improving insulin sensitivity, inhibiting glucose-degrading enzymes, to directly binding with insulin receptors(3). With various blood sugar regulation mechanisms, bitter melon has gained significant attention in the field of blood sugar control. However, research shows that if specific component structures are not extracted or if the product is only following market trends and not adequately utilized, over time, ineffective feedback from consumers may lead to market saturation, causing high-quality products to be overlooked.
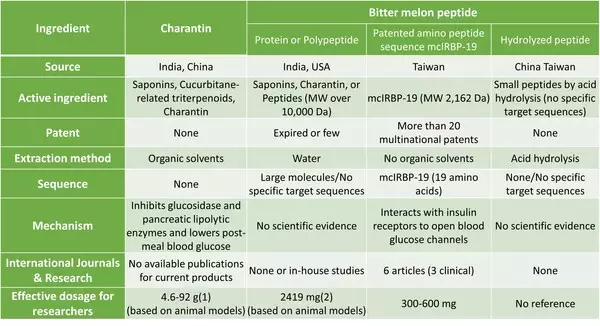
Research on Commercial Bitter Melon Health Ingredients
Research on Patented Amino Peptide Sequence mcIRBP-19
In an environment saturated with products claiming to contain bitter melon peptides, many of these lack scientific validation of their functionality or physiological mechanisms. The market is chaotic, with products of varying quality. Due to the complex mechanism of blood sugar regulation, there is rarely a structure that can substitute for insulin in regulating blood sugar. It has been traditionally believed that small molecular peptides or large proteins with high absorption rates cannot bind to insulin receptors, thus their effectiveness is limited. However, Greenyn Biotechnology is still committed to in conducting in-depth research on bitter melon, focusing on the essential nature of the plant. They have verified that the specific amino peptide sequence mcIRBP-19, consisting of 19 amino acids, is a rare natural substance that can directly bind to the diabetes-causing target - the insulin receptor. They have completed structural sequencing and quantitative analysis, providing scientific evidence that repeatedly confirms its unique physiological function. Additionally, they have conducted multiple in vivo functional studies, which are detailed as follows:
Regulating Blood Sugar and Assisting in the Treatment of Diabetes
Diabetes is an irreversible disease, and its alarming annual increase rate of 25,000 cases is not the only cause for concern. The complications it leads to, such as retinopathy, neuropathy, and cardiovascular diseases, are even more significant.
Through animal experiments analyzing fasting blood sugar and post-meal blood sugar regulation capabilities, it was found that the patented amino peptide sequence 19 (mcIRBP-19) can reduce fasting blood sugar by 50% and glycosylated hemoglobin by 38%. Additionally, it maintains a balanced insulin level in the body. Furthermore, it prevents a significant increase in post-meal blood sugar, rapidly regulating blood sugar within 2 hours. Its exceptional ability to stabilize blood sugar can significantly reduce the occurrence of complications caused by diabetes, such as retinopathy.
Human clinical studies have shown (4) that the patented amino peptide sequence 19 (mcIRBP-19) can assist in significantly reducing fasting blood sugar and glycosylated hemoglobin within 12 weeks for individuals who have shown insufficient improvement with conventional diabetes medication. It can also effectively improve the quality of life for patients (5). For sub-healthy individuals with diabetes, mcIRBP-19 can help regulate fasting blood sugar, stabilize glycated hemoglobin, and prevent the onset of diabetes. These clinical trials have verified the potential efficacy of this ingredient
Reducing Body Fat, Blood Lipids, and Anti-Inflammation
In addition to unstable blood sugar, metabolic syndrome also involves issues with abnormal blood lipids, central obesity, and chronic inflammation. Animal experiments (6) have shown that the patented amino peptide sequence 19 (mcIRBP-19) can regulate fatty acid metabolism and genes related to fatty liver. It effectively reduces body fat percentage by 25.5%, significantly decreasing white fat in the liver. This helps prevent the occurrence of fatty liver and avoids liver damage. Furthermore, chronic inflammation, much like an internal fire, can lead to various diseases, including cancer. The patented sequence 19 peptide can effectively reduce the expression of inflammatory factors in various organs, thus preventing the onset of chronic diseases.
Conclusion
The human body is like a machine. When metabolic syndrome occurs, it's like screws gradually loosening. Continued operation can easily lead to machine failure, resulting in conditions such as chronic diseases and cancer. At such times, we urgently need a helper who can tighten the screws.
The structure of mcIRBP-19, with the potential of a plant-derived insulin, is extracted using patented technology. The functional 19 amino acids are fully sequenced. Through a series of animal and clinical experiments, it has been confirmed to stabilize both pre-meal and post-meal blood sugar levels, reduce body fat and blood lipids, and achieve anti-inflammatory effects while providing adjunctive treatment for diabetes. This not only brings a ray of hope to diabetes patients, preventing the deterioration of their condition, but also takes into account their quality of life. It offers everyone a safe and effective natural preventive health option for daily life.
Reference
1. Wang HY, et al. Food Chem Toxicol. (2014) 69:347-356.
2. Dallas LC, et al. J Med Food. (2011) 14:1496-1504.
3. Lo HY, et al. J Agric Food Chem. (2013) 61:2461-2468.
4. Hsu PK, et al. Nutrients (2020) 12:1252.
5. Pan F, et al. Risk Manag Healthc Policy. (2020) 13:2219-2226.
6. Patent Invention No. I580690, Republic of China.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Plant Insulin - mcIRBP-19: Natural Key to Blood Sugar Regulation