INCHEON, South Korea, Oct. 14, 2024 /PRNewswire/ -- Samsung Biologics (KRX: 207940.KS), a global contract development and manufacturing organization (CDMO), launched today a new high-concentration formulation platform to support the development and manufacturing of high-dose biopharmaceuticals.
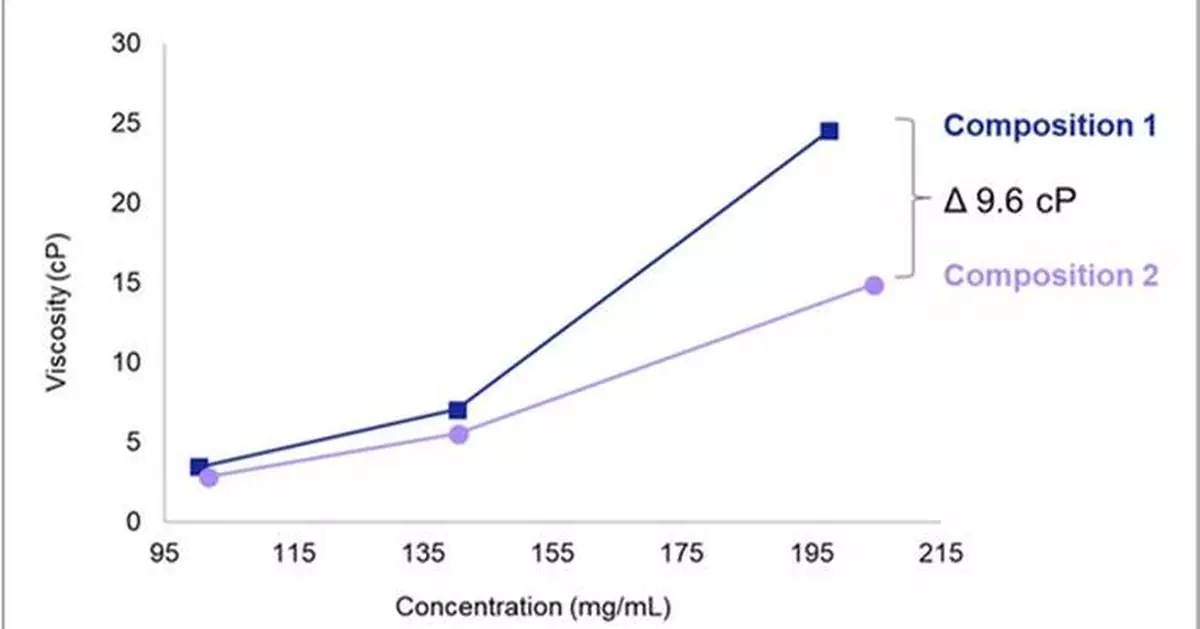
S-HiConTM can proactively identify unintended pH changes, enhance formulation stability, and reduce viscosity to ensure efficacy and maximize drug delivery. Through optimization of pH, buffer species, and excipients, along with a preliminary 'Concentration Gate Check' process, the platform tests formulation feasibility in the initial stages to identify favorable candidates and minimize potential risks associated with high concentration development. Additionally, S-HiConTM integrates analytical capabilities to ensure that critical formulation challenges associated with viscosity and protein aggregation are addressed (Figure 1). Samsung Biologics has recorded over 200 mg/mL for liquid formulations, with adequate viscosity and improved stability.
"Our new platform will enable us to provide innovative solutions for clients requiring low to ultra-high concentration formulation to develop advanced therapeutics," said Brian Hosung Min, Executive Vice President and Head of CDO Development. "Samsung Biologics is committed to providing customized services by leveraging our expertise and track record in contract development."
The latest offering is part of Samsung Biologics' continued efforts to provide clients with innovative technologies that enable high-quality development. Leveraging platforms that can increase antibody-dependent cellular cytotoxity (ADCC) activity and enhance upstream process quality, the company is expected to better accommodate clients' evolving needs and support their pipelines. Visit our website to learn more about our CDO platforms: https://samsungbiologics.com/services/cdo/overview
About Samsung Biologics Co., Ltd.
Samsung Biologics (KRX: 207940.KS) is a fully integrated, end-to-end CDMO service provider, offering seamless development and manufacturing solutions from cell line development to final aseptic fill/finish as well as laboratory testing support for the biopharmaceutical products we manufacture. Our state-of-the-art facilities are CGMP compliant with bioreactors ranging from small to large scales to serve varying client needs. To maximize our operational efficiency and expand our capabilities in response to growing biomanufacturing demand, Samsung Biologics completed Bio Campus I with Plant 4, offering a combined 604 kL total capacity, and launched Bio Campus II with the construction of Plant 5, which will be operational in April 2025, adding 180 kL biomanufacturing capacity. Additionally, Samsung Biologics America enables the company to work in closer proximity to clients based in the U.S. and Europe. We continue to upgrade our capabilities to accommodate our clients by investing in technologies such as an antibody-drug conjugate (ADC) facility, a dedicated mRNA manufacturing facility, and additional aseptic filling capacity. As a sustainable CDMO partner of choice, we are committed to on-time, in-full delivery of the products we manufacture with our flexible manufacturing solutions, operational excellence, and proven expertise.
Samsung Biologics Media Contact
Claire Kim, Head of Global Marketing Communications
cair.kim@samsung.com
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Samsung Biologics launches high-concentration formulation platform to accelerate high-dose drug development