LONDON (AP) — Lawyers for convicted baby killer Lucy Letby and former executives at the hospital where she worked have asked to halt an inquiry into the deaths after a panel of medical experts found no evidence of a crime, a judge said Monday.
Justice Kathryn Thirlwall announced the requests before hearing closing statements in the inquiry seeking accountability for staff and management for the harm to babies at Countess of Chester Hospital in northwest England.
The inquiry that began in September was predicated on Letby's guilt, and Thirlwall had said she would not review the convictions after an appeals court upheld them. But lawyers for Letby said that if the convictions are overturned, the inquiry might reach the wrong conclusions, and 10 million pounds ($13 million) spent so far will have been a waste of taxpayer funds.
“In short, it will defeat the purpose of a public inquiry, to fully and fearlessly understand the circumstances in which the babies died or became unwell," attorney Louise Mortimer wrote in a letter to Thirlwall.
Thirlwall is expected to publish a final report in the fall.
Letby, 35, is serving multiple life sentences with no chance of release after being convicted of seven counts of murder and attempting to murder seven other infants between June 2015 and June 2016 while working as a neonatal nurse at the hospital.
Prosecutors said Letby left little or no trace when she harmed babies: injecting air into their bloodstreams, administering air or milk into their stomachs via nasogastric tubes, interfering with breathing tubes or poisoning them with insulin. They said she was the only employee on duty in the neonatal unit when the children collapsed or died.
But a group of 16 international medical experts in pediatrics and neonatology who reviewed the medical evidence concluded that natural causes or bad medical care led to the death or collapse of each newborn, Dr. Shoo Lee, a retired neonatologist from Canada, said last month.
The panel also questioned the premise that there was an unexplained spike in deaths at the hospital. It said evidence of schedules that showed Letby was present during all the deaths was “incomplete, selective and, therefore, meaningless."
Dozens of Letby's supporters gathered outside the inquiry in Liverpool to call for her release. They held signs saying: “Free Lucy Letby! No babies were murdered!” and others referring to flawed science and the “nurse Letby witch hunt.”
Letby’s convictions are being examined by the Criminal Case Review Commission, which looks at potential miscarriages of justice. It could refer its findings to the Court of Appeal.
The Crown Prosecution Service has said two juries convicted Letby and three appellate judges had rejected her arguments that the prosecution expert evidence was flawed.
Former executives at the hospital — chief executive Tony Chambers, medical director Ian Harvey, director of nursing Alison Kelly and human resources director Sue Hodkinson — asked for the public inquiry to be halted after the medical panel released its findings.
But a lawyer for the National Health Service trust that runs the hospital said during his closing remarks that the inquiry shouldn't be suspended.
“A postponement, which would necessarily be of indeterminate length, is not warranted and it would serve to delay the implementations of recommendations which unhappily the evidence in this inquiry have demonstrated are desperately needed,” attorney Andrew Kennedy said.
Kennedy said the hospital had acknowledged some failings at the start of the inquiry but that it was now clear it should have acted even sooner to banish Letby from the neonatal unit.
“We recognize the significance of this concession to the parents of children who were harmed or killed by Letby after the beginning of November 2015 and I can only say that the trust is profoundly sorry for the failure to intervene sooner," Kennedy said.
Several related investigations remain active.
Cheshire police announced last week that its investigation into corporate manslaughter at the hospital had been expanded and was now looking at individual suspects for gross negligence manslaughter.
Letby's lawyer criticized that decision, based on the medical panel's findings.
“We now have substantial and significant expert evidence which completely demolishes the prosecution case against Lucy Letby and points the finger in a very different direction to that which the police are currently looking,” Mark McDonald said. "It is time they take a step back and ask themselves whether have they made a huge mistake.”
A separate investigation is looking into other deaths and near-deaths of babies at the Countess of Chester Hospital and the Liverpool Women’s Hospital when Letby worked there between 2012 to 2016.
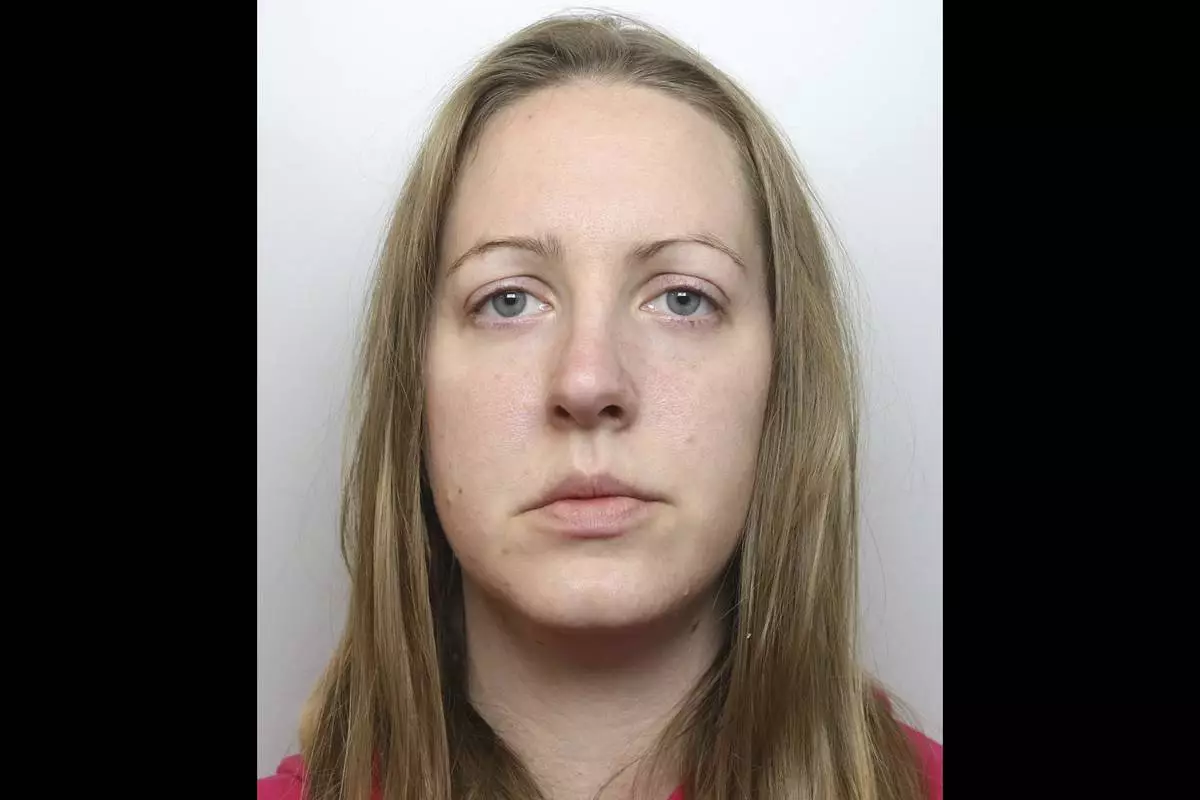
FILE- This undated handout issued by Cheshire Constabulary shows of nurse Lucy Letby. (Cheshire Constabulary via AP, file)