Avenzo to receive exclusive global license (excluding Greater China) for development, manufacturing and commercialization of potential best-in-class EGFR/HER3 antibody-drug conjugate
EGFR and HER3 co-expressed across various solid tumors, including non-small cell lung cancer, breast cancer, and head and neck cancer
First-in-human clinical study expected this year
SAN DIEGO, SOMERSET, N.J. and SHANGHAI, Jan. 7, 2025 /PRNewswire/ -- Avenzo Therapeutics, Inc. ("Avenzo"), a clinical-stage biotechnology company developing next-generation oncology therapies, and Duality Biotherapeutics ("DualityBio"), a clinical-stage biotech company focusing on the discovery and development of next-generation antibody-drug conjugate (ADC) therapeutics, today announced that they have entered into an exclusive license agreement, pursuant to which Avenzo will develop, manufacture and commercialize AVZO-1418/DB-1418, a potential best-in-class EGFR/HER3 bispecific ADC, globally (excluding Greater China).
"DualityBio has a strong track record of developing and advancing a pipeline of differentiated ADCs that target a broad range of indications," said Athena Countouriotis, M.D., Co-founder, President and CEO of Avenzo Therapeutics. "By targeting both EGFR and HER3 with its differentiated design, AVZO-1418/DB-1418 has the potential to provide clinical benefit across multiple solid tumor types. We are excited to add this drug candidate to our clinical pipeline and look forward to collaborating with DualityBio to quickly progress this program into clinical studies."
Under the terms of the agreement, DualityBio will receive an upfront payment of $50 million and will be eligible to receive up to approximately $1.15 billion in development, regulatory and commercial milestone payments. In addition, DualityBio is eligible to receive tiered royalties on sales in Avenzo's territory.
"DualityBio and Avenzo share a common purpose in developing the next generation of oncology therapies that address underserved therapeutic areas," said John Zhu, Ph.D., Founder and CEO of DualityBio. "Based on preclinical studies, we believe AVZO-1418/DB-1418 has the potential to offer enhanced therapeutic benefits over other therapies. We look forward to partnering with the Avenzo team to accelerate the development of this program and provide a potential new treatment option to cancer patients."
IND-enabling studies are ongoing with plans to advance AVZO-1418/DB-1418 into a first-in-human clinical study this year.
About AVZO-1418/DB-1418
AVZO-1418/DB-1418 is an EGFR/HER3 dual targeting, topoisomerase-1 inhibitor-based ADC built from DualityBio's proprietary Duality Innovative Bispecific Antibody Conjugate (DIBAC) platform. In preclinical studies, AVZO-1418/DB-1418 has exhibited higher binding affinity to tumor cells and has demonstrated its efficacy potential across various solid tumors, including those that are EGFR-resistant, EGFR-low or HER3-resistant.
About Avenzo Therapeutics
Avenzo Therapeutics is a clinical-stage biotechnology company focused on developing next-generation oncology therapies for patients. The company's lead drug candidate, AVZO-021, is a novel, highly potent and selective inhibitor of CDK2, a key enzyme involved in cell cycle regulation. AVZO-021 is being studied in a Phase 1 study in the U.S. for the treatment of advanced solid tumors and in combinations in HR+/HER2-negative metastatic breast cancer. Avenzo is headquartered in San Diego, California. For more information, visit us at www.avenzotx.com or on LinkedIn.
About DualityBio
Duality Biotherapeutics is a clinical-stage biotech company dedicated to the discovery and development of next-generation ADCs to treat cancer and autoimmune diseases. DualityBio has successfully built several cutting-edge ADC technology platforms with global intellectual property rights. Leveraging a robust pipeline, DualityBio is conducting multiple global clinical trials across 17 countries and has enrolled over 1,500 patients for multiple clinical-stage ADC candidates.
Additionally, DualityBio has established strategic collaborations with global MNCs and leading biotech innovators. As a global ADC powerhouse, DualityBio is developing "Super ADCs," including bispecific ADCs, novel-payload ADCs, and autoimmune ADCs. For more information, please visit www.dualitybiologics.com.
Avenzo Therapeutics Contact:
Carla Taub
Media Relations
ctaub@avenzotx.com
DualityBio Media Contact:
PR@dualitybiologics.com
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Avenzo Therapeutics and DualityBio Announce Exclusive Global License for Potential Best-In-Class EGFR/HER3 Antibody-Drug Conjugate
SINGAPORE, April 8, 2025 /PRNewswire/ -- Lazada, a leading eCommerce platform in Southeast Asia, today published its research report on online sellers, Bridging the AI Gap: Online Seller Perceptions and Adoption Trends in Southeast Asia. Developed in collaboration with Kantar, the report surveyed 1,214 eCommerce sellers across Indonesia, Malaysia, the Philippines, Singapore, Thailand, and Vietnam to examine AI adoption trends, challenges, and opportunities—shedding light on sellers' readiness to integrate AI into their operations.
Knowledge, perception and implementation gap evident among online sellers
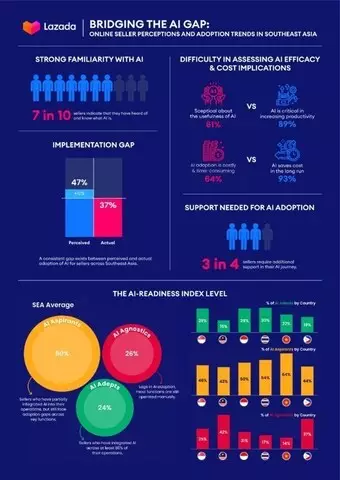
The research reveals that online sellers in Southeast Asia show strong familiarity of AI, with nearly seven in 10 sellers (68%) indicating that they are aware of AI. Sellers believe they have integrated AI into 47% of their business operations, even though actual adoption stands only at 37%, highlighting a clear gap between perceived and actual AI adoption.
Online sellers face a dilemma in terms of assessing AI efficacy and its cost implications. While 89% acknowledge AI's role in boosting productivity, almost two-third (61%) remain sceptical about its overall usefulness. Furthermore, although nearly all sellers (93%) agree that AI can drive long-term cost savings, 64% cite costliness and time-consuming implementation as barriers to adoption.
The research also suggests an implementation gap, where sellers understand the importance of AI but struggle with effective deployment. Highlighting the challenge of transitioning from familiar, manual processes to AI-driven solutions, nearly all sellers (93%) agree that it is important to upskill the workforce to use AI so that they can be more productive, yet 3 out of 4 sellers (75%) also concede that their employees still prefer to use tools they are familiar with, rather than new AI solutions.
AI-readiness level varies across markets
Across the region, Indonesia and Vietnam lead with 42% AI adoption across business functions, while Singapore and Thailand follow closely at 39%. Based on the level of AI adoption across five core aspects of operations of a seller's business, namely operations and logistics, product management, marketing and advertising, customer service, and workforce management, the report identifies three distinct seller archetypes – AI Adepts, AI Aspirants, and AI Agnostics[1], based on the average score they attained in each aspect of operations to represent their readiness level to embrace AI:
Findings indicate that Thailand has the highest share of AI Adepts, with 30% of sellers in this category. Singapore (29%), Indonesia (29%), and Vietnam (22%) also demonstrate strong AI implementation despite knowledge gaps, while Malaysia (15%) and the Philippines (19%) face challenges related to internal buy-in and infrastructure limitations. With the majority of Southeast Asian sellers (76%) falling within the AI Aspirants and AI Agnostics categories, they are actively seeking more effective AI-powered solutions, with high demand for AI-powered tools (42%) and enhanced seller support (41%).
"The findings from our research reveal a fascinating gap in Southeast Asia's eCommerce ecosystem. While most sellers understand AI's transformative potential, many are still navigating the path from recognition to implementation," said James Dong, Chief Executive Officer, Lazada Group. "As a leading eCommerce platform in Southeast Asia, we aim to bridge the knowledge and adoption gap by developing accessible AI solutions that address the unique challenges faced by sellers across different markets, ultimately making technology more accessible and driving sustainable business growth regardless of a seller's size or technical expertise."
Leveraging Lazada's AI-driven solutions to transform business operations
To support sellers in their AI adoption journey, Lazada is launching the Online Sellers Artificial Intelligence Readiness Playbook, designed to provide strategic guidance based on sellers' AI maturity levels. The research reveals that sellers are already leveraging key AI-driven solutions on Lazada's platform to enhance their efficiency, validating Lazada's continuous investments into cutting-edge AI innovations and advanced tools that streamline eCommerce operations and drive competitiveness.
With 67% of sellers expressing strong satisfaction in existing Lazada AI features[2], Lazada is also releasing new Generative AI (GenAI) features that are designed to empower sellers and enhance their product listings, streamline operations, and boost customer conversions such as:
To find out more, download the Online Sellers Artificial Intelligence Readiness Playbook to understand how these solutions can offer a structured framework for sellers to integrate AI into their workflows to drive growth, efficiency, and innovation in an ever-evolving eCommerce landscape.
[1] AI Adepts are sellers in the top 25th percentile for AI adoption in terms of their scores, AI Aspirants fall within the middle 25th and 75th percentile, and AI Agnostics are in the bottom 25th percentile.
[2] Refer to Annex for more details
About the Research
Developed in partnership with Kantar, Bridging the AI Gap: Online Seller Perceptions and Adoption Trends in SEA provides a comprehensive analysis of AI adoption trends, challenges, and opportunities, offering insights into how sellers can leverage AI to drive growth and efficiency in Southeast Asia's evolving eCommerce landscape. The report surveyed 1,214 eCommerce sellers across Indonesia, Malaysia, the Philippines, Singapore, Thailand, and Vietnam in February 2025.
This follows the Artificial Intelligence Adoption in eCommerce in Southeast Asia Whitepaper, another research conducted in partnership with Kantar, which surveyed more than 6,000 eCommerce users in the region in September 2024 to better understand AI awareness, trust and preferences, shopping behaviour, and consumer pain points in the region.
About Lazada Group
Lazada Group is Southeast Asia's pioneer eCommerce platform. For the last 13 years, Lazada has been accelerating progress in Indonesia, Malaysia, the Philippines, Singapore, Thailand and Vietnam through commerce and technology. Today, a thriving local ecosystem links about 160 million active users to more than one million actively-selling sellers every month, who are transacting safely and securely via trusted payment channels and Lazada Wallet, receiving parcels through a homegrown logistics network that has become the largest in the region.
Annex
Lazada's AI features for sellers:
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Three in Four Southeast Asian ECommerce Sellers Require Additional Support in Their AI Adoption, Lazada Report Reveals